有机化学 ›› 2024, Vol. 44 ›› Issue (6): 1907-1919.DOI: 10.6023/cjoc202311027 上一篇 下一篇
研究论文
胡懿鸣a,†, 许嘉宇a,†, 汤敏a, 刘雅雯a, 关丽萍a,*( ), 金晴昊b,*(
), 金晴昊b,*( )
)
收稿日期:2023-11-24
修回日期:2024-01-22
发布日期:2024-03-12
作者简介:基金资助:
Yiming Hua,†, Jiayu Xua,†, Min Tanga, Yawen Liua, Liping Guana,*( ), Qinghao Jinb,*(
), Qinghao Jinb,*( )
)
Received:2023-11-24
Revised:2024-01-22
Published:2024-03-12
Contact:
* E-mail: About author:Supported by:文章分享

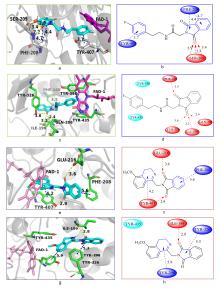
合成了一系列2-(1,3-二氧代异吲哚啉-2-基)-N-苯乙酰胺和2-(3,4-二氢异喹啉-1-基)异吲哚-1,3-二酮衍生物, 并评价了它们抑制单胺氧化酶(MAO)和胆碱酯酶(ChE)生物活性. 实验结果显示, 所有的化合物对单胺氧化酶具有一定的抑制活性. 再分别进行单胺氧化酶A (MAO-A)和单胺氧化酶B (MAO-B)抑制活性研究, 发现所有化合物对MAO-B显示比较好的抑制活性, 对MAO-A显示弱的抑制活性. 其中, 2-(1,3-二氧代异吲哚-2-基)-N-(4-氟苯基乙基)乙酰胺(2h)和2-((7-甲氧基-3,4-二氢异喹啉-1-基)甲基)异吲哚-1,3-二酮(3d)显示最好的抑制MAO-A和MAO-B作用, 对MAO的IC50值分别为(3.87±0.59)和(3.35±0.53) μmol/L. 细胞毒性实验结果显示, 抑制活性比较好的化合物对L929细胞没有细胞毒性. 化合物2h和3d分子对接的结果表明, 化合物2h和3d与MAO-A和MAO-B之间存在明显的相互作用. 另外, 所有的化合物对乙酰胆碱酯酶(AChE)和丁酰胆碱酯酶(BuChE)显示比较弱的抑制活性.
胡懿鸣, 许嘉宇, 汤敏, 刘雅雯, 关丽萍, 金晴昊. 2-(1,3-二氧代异吲哚啉-2-基)-N-苯乙酰胺和2-(3,4-二氢异喹啉-1-基)异吲哚-1,3-二酮类单胺氧化酶(MAO)和胆碱酯酶(ChE)抑制剂的设计、合成和生物活性研究[J]. 有机化学, 2024, 44(6): 1907-1919.
Yiming Hu, Jiayu Xu, Min Tang, Yawen Liu, Liping Guan, Qinghao Jin. Design, Synthesis and Biological Activity Studies of 2-(1,3-Dioxoiso-indolin-2-yl)-N-phenethylacetamide and 2-(3,4-Dihydroisoquinolin-1-yl)isoindole-1,3-dione as Monoamine Oxidase (MAO) and Cholinesterase (ChE) Inhibitors[J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1907-1919.
| Compd. | R | Inhibition rate/% | IC50/(μmol•L–1) |
|---|---|---|---|
| 2a | H | 67.59 | 6.00±0.77 |
| 2b | 4-CH3 | 62.21 | 4.42±0.61 |
| 2c | 2-OCH3 | 41.83 | 14.03±1.14 |
| 2d | 4-OCH3 | 64.21 | 4.09±0.61 |
| 2e | 3,4-(OCH3)2 | 58.82 | 5.23±0.72 |
| 2f | 2-F | 41.83 | 14.73±1.16 |
| 2g | 3-F | 68.63 | 3.64±0.56 |
| 2h | 4-F | 66.01 | 3.87±0.59 |
| 2i | 4-Cl | 86.27 | 6.34±0.95 |
| 2j | 2-Br | 60.13 | 6.18±0.79 |
| 2k | 3-Br | 65.36 | 4.94±0.60 |
| 2l | 4-Br | 35.95 | 15.93±1.45 |
| 3a | H | 80.66 | 5.29±2.24 |
| 3b | 4-CH3 | 61.56 | 4.55±0.60 |
| 3c | 2-OCH3 | 38.57 | 13.5±1.55 |
| 3d | 4-OCH3 | 91.98 | 3.35±0.53 |
| 3e | 3,4-(OCH3)2 | 52.72 | 9.40±1.76 |
| 3f | 2-F | 63.68 | 4.17±0.64 |
| 3g | 3-F | 79.25 | 3.91±0.60 |
| 3h | 4-F | 96.70 | 1.53±0.19 |
| 3i | 4-Cl | 79.48 | 3.94±1.43 |
| 3j | 2-Br | 87.27 | 3.10±0.36 |
| 3k | 3-Br | 76.42 | 4.86±0.69 |
| 3l | 4-Br | 50.47 | 9.58±0.44 |
| Rasagiline | — | 88.30 | 1.68±0.75 |
| Compd. | R | Inhibition rate/% | IC50/(μmol•L–1) |
|---|---|---|---|
| 2a | H | 67.59 | 6.00±0.77 |
| 2b | 4-CH3 | 62.21 | 4.42±0.61 |
| 2c | 2-OCH3 | 41.83 | 14.03±1.14 |
| 2d | 4-OCH3 | 64.21 | 4.09±0.61 |
| 2e | 3,4-(OCH3)2 | 58.82 | 5.23±0.72 |
| 2f | 2-F | 41.83 | 14.73±1.16 |
| 2g | 3-F | 68.63 | 3.64±0.56 |
| 2h | 4-F | 66.01 | 3.87±0.59 |
| 2i | 4-Cl | 86.27 | 6.34±0.95 |
| 2j | 2-Br | 60.13 | 6.18±0.79 |
| 2k | 3-Br | 65.36 | 4.94±0.60 |
| 2l | 4-Br | 35.95 | 15.93±1.45 |
| 3a | H | 80.66 | 5.29±2.24 |
| 3b | 4-CH3 | 61.56 | 4.55±0.60 |
| 3c | 2-OCH3 | 38.57 | 13.5±1.55 |
| 3d | 4-OCH3 | 91.98 | 3.35±0.53 |
| 3e | 3,4-(OCH3)2 | 52.72 | 9.40±1.76 |
| 3f | 2-F | 63.68 | 4.17±0.64 |
| 3g | 3-F | 79.25 | 3.91±0.60 |
| 3h | 4-F | 96.70 | 1.53±0.19 |
| 3i | 4-Cl | 79.48 | 3.94±1.43 |
| 3j | 2-Br | 87.27 | 3.10±0.36 |
| 3k | 3-Br | 76.42 | 4.86±0.69 |
| 3l | 4-Br | 50.47 | 9.58±0.44 |
| Rasagiline | — | 88.30 | 1.68±0.75 |
| Compd. | R | Inhibition rate/% | IC50/(μmol•L–1) of MAO-B | |
|---|---|---|---|---|
| MAO-A | MAO-B | |||
| 2a | H | 58.06 | 76.80 | 5.20±1.83 |
| 2b | 4-CH3 | 22.58 | 65.46 | 7.27±0.86 |
| 2c | 2-OCH3 | 42.42 | 38.85 | |
| 2d | 4-OCH3 | 32.26 | 61.60 | 7.47±1.62 |
| 2e | 3,4-(OCH3)2 | 22.64 | 78.98 | 5.58±0.75 |
| 2f | 2-F | 21.21 | 44.94 | |
| 2g | 3-F | 20.97 | 30.15 | |
| 2h | 4-F | 64.52 | 75.26 | 5.02±0.70 |
| 2i | 4-Cl | 29.03 | 63.14 | 7.36±1.75 |
| 2j | 2-Br | 66.04 | 10.83 | |
| 2k | 3-Br | 3.23 | 30.41 | |
| 2l | 4-Br | 60.61 | 31.44 | |
| 3a | H | 31.25 | 1.37 | |
| 3b | 4-CH3 | 34.38 | 21.92 | |
| 3c | 2-OCH3 | 69.7 | 69.59 | 7.41±2.76 |
| 3d | 4-OCH3 | 78.75 | 89.04 | 4.82±0.83 |
| 3e | 3,4-(OCH3)2 | 27.42 | 74.23 | 4.94±0.69 |
| 3f | 2-F | 28.13 | 8.90 | |
| 3g | 3-F | 25.00 | 71.23 | 4.84±1.25 |
| 3h | 4-F | 15.63 | 35.65 | |
| 3i | 4-Cl | 4.69 | 72.60 | 4.86±1.35 |
| 3j | 2-Br | 21.88 | 49.32 | |
| 3k | 3-Br | 34.38 | 78.08 | 5.40±0.73 |
| 3l | 4-Br | 26.56 | 35.62 | |
| Compd. | R | Inhibition rate/% | IC50/(μmol•L–1) of MAO-B | |
|---|---|---|---|---|
| MAO-A | MAO-B | |||
| 2a | H | 58.06 | 76.80 | 5.20±1.83 |
| 2b | 4-CH3 | 22.58 | 65.46 | 7.27±0.86 |
| 2c | 2-OCH3 | 42.42 | 38.85 | |
| 2d | 4-OCH3 | 32.26 | 61.60 | 7.47±1.62 |
| 2e | 3,4-(OCH3)2 | 22.64 | 78.98 | 5.58±0.75 |
| 2f | 2-F | 21.21 | 44.94 | |
| 2g | 3-F | 20.97 | 30.15 | |
| 2h | 4-F | 64.52 | 75.26 | 5.02±0.70 |
| 2i | 4-Cl | 29.03 | 63.14 | 7.36±1.75 |
| 2j | 2-Br | 66.04 | 10.83 | |
| 2k | 3-Br | 3.23 | 30.41 | |
| 2l | 4-Br | 60.61 | 31.44 | |
| 3a | H | 31.25 | 1.37 | |
| 3b | 4-CH3 | 34.38 | 21.92 | |
| 3c | 2-OCH3 | 69.7 | 69.59 | 7.41±2.76 |
| 3d | 4-OCH3 | 78.75 | 89.04 | 4.82±0.83 |
| 3e | 3,4-(OCH3)2 | 27.42 | 74.23 | 4.94±0.69 |
| 3f | 2-F | 28.13 | 8.90 | |
| 3g | 3-F | 25.00 | 71.23 | 4.84±1.25 |
| 3h | 4-F | 15.63 | 35.65 | |
| 3i | 4-Cl | 4.69 | 72.60 | 4.86±1.35 |
| 3j | 2-Br | 21.88 | 49.32 | |
| 3k | 3-Br | 34.38 | 78.08 | 5.40±0.73 |
| 3l | 4-Br | 26.56 | 35.62 | |

| [1] |
Stefanie, A.; Iyengar, R. E.; Rebecca, S.; Bencie, N. M.; Jeenu, A. M.; Rahul, E.; Adrien, A. J. Drug targeting. 2020, 28, 10.
|
| [2] |
Lehn, A.; Gelauff, J.; Hoeritzauer, I.; Ludwig, L.; McWhirter, L.; Williams, S.; Gardiner, P.; Carson, A.; Stone, J. J. Neurol. 2016, 263, 611.
|
| [3] |
Mishra, A.; Bandopadhyay, R.; Singh, P. K.; Mishra, P. S.; Sharma, N.; Khurana, N. Metab. Brain Dis. 2021, 36, 1591.
|
| [4] |
Furmark, T. Isr. Ann. Psychiatry Relat. Discip. 2009, 46, 5.
|
| [5] |
Lane, C. A.; Hardy, J.; Schott, J. M. Eur. J. Neurol. 2018, 25, 59.
doi: 10.1111/ene.13439 pmid: 28872215 |
| [6] |
Mantzavinos, V.; Alexiou, A. Curr. Alzheimer Res. 2017, 14, 1149.
doi: 10.2174/1567205014666170203125942 pmid: 28164766 |
| [7] |
Weller, J.; Budson, A. Eur. J. Neurol. 2019, 5, 43.
|
| [8] |
Li, Y. Y.; Tang, Q.; Zheng, L. F.; He, D.; Jian, S.; Zhang, M. Eur. J. Med. Chem. 2020, 166, 49.
|
| [9] |
Xing, S.; Sun, H. P. Pharm. Prog. 2023, 47, 179. (in Chinese)
|
|
(邢帅帅, 孙昊鹏, 药学进展, 2023, 47, 179.)
|
|
| [10] |
Marucci, G.; Buccioni, M.; Ben, D. D.; Lambertucci, C.; Volpinim, R.; Amenta, F. J. Neurol. 2021, 190, 108352.
|
| [11] |
Anand, P.; Singh, B. Arch. Pharm. Res. 2013, 36, 375.
|
| [12] |
Caraci, F.; Copani, A.; Nicoletti, F.; Drago, F. Eur. J. Pharmacol. 2010, 626, 64.
|
| [13] |
Tune, L. E. Eur. J. Pharmacol. 2008, 8, 91.
|
| [14] |
Liu, Y. H.; Xu, H. D.; Dong, X. H.; Zhang, N.; Luo, X. T.; Zhang, H.; Liu, B. Chin. Herb. Med. 2023, 7, 1836. (in Chinese)
|
|
(刘彦宏, 徐红丹, 董晓红, 张宁, 罗孝廷, 张红, 刘斌, 中药材, 2023, 7, 1836.)
|
|
| [15] |
Liu, F.; Zhou, Y. J. Clin. Psychosom. Dis. 2007, 3, 251. (in Chinese)
|
|
(刘峰, 周艳, 临床心身疾病杂志, 2007, 3, 251.)
|
|
| [16] |
Bhawna-Kumar, A.; Bhatia, M.; Kapoor, A.; Kumar, P.; Kumar, S. Eur. J. Med. Chem. 2022, 242, 114655.
|
| [17] |
Özdemir, Z.; Alagöz, M. A.; Bahçecioğlu, Ö. F.; Gök, S. Curr. Med. Chem. 2021, 28, 6045.
doi: 10.2174/0929867328666210203204710 pmid: 33538661 |
| [18] |
Carradori, S.; Secci, D.; Petzer, J. P. Expert Opin. Ther. Pat. 2018, 3, 211.
|
| [19] |
Zhang, N. Med. Theory Pract. 2015, 28, 1713. (in Chinese)
|
|
(张娜, 医学理论与实践, 2015, 28, 1713.)
|
|
| [20] |
Mzezewa, S. C.; Omoruyi, S. I.; Zondagh, L. S.; Malan, S. F.; Ekpo, O. E.; Joubert, J. J. Enzyme Inhib. Med. Chem. 2021, 36, 1607.
|
| [21] |
Sang, Z. P.; Song, Q.; Cao, Z. C.; Deng, Y.; Zhang, L. J. Enzyme Inhib. Med. Chem. 2022, 37, 69.
|
| [22] |
Das, T.; Saha, S. C.; Sunita, K. S. Afr. J. Bot. 2022, 11, 146.
|
| [23] |
Zhang, H. H. M.S. Thesis, Lanzhou University, Lanzhou, 2022. (in Chinese)
|
|
(张红花, 硕士论文, 兰州大学, 兰州, 2022.)
|
|
| [24] |
Unzeta, M.; Esteban, G.; Bolea, I.; Fogel, W. A.; Ramsay, R. R.; Youdim, M. B.; Tipton, K. F.; Marco-Contelles, J. Front. Neurosci. 2016, 10, 205.
|
| [25] |
Zhang, M.; Zhang, H.; Zheng, J. X.; Mo, H.; Xia, K. F.; Jian, S. G. Int. J. Mol. Sci. 2018, 19, 3446.
|
| [26] |
Plazas, E.; Avila, M. C.; Muñoz, D. R.; Cuca, S. L. E. Pharmacol. Res. 2022, 177, 106126.
|
| [27] |
Zou, X. H.; Fu, X. Q.; Wang, W. W. Cardiovasc. Dis. J. Integr. Tradit. Chin. West Med. 2023, 21, 3497. (in Chinese)
|
|
(邹旭欢, 付雪琴, 王玮玮, 中西医结合心脑血管病杂志, 2023, 21, 3497.)
|
|
| [28] |
Bonnet, U. CNS Drug Rev. 2003, 9, 140.
|
| [29] |
Behlke, L. M.; Lenze, E. J.; Carney, R. M. CNS Drugs 2020, 34, 1133.
|
| [30] |
Liang, Y.; Zhang, L.; Zeng, Z. C. Chin. J. New Drugs 2018, 27, 1603. (in Chinese)
|
|
(梁瑶, 张蕾, 曾灶昌, 中国新药杂志, 2018, 27, 1603.)
|
|
| [31] |
Pan, S. L.; Xie, J.; Qian, F. G.; Wang, J.; Shao, Y. C. Acta Pharm. Sin. 2005, 4, 355. (in Chinese)
|
|
(潘胜利, 解静, 钱伏刚, 王峻, 邵衣慈, 药学学报, 2005, 4, 355.)
|
|
| [32] |
Verma, S.; Lal, S.; Narang, R.; Sudhakar, K. Chem. Med. Chem. 2023, 18, e202200571.
|
| [33] |
Teng, P.; Li, C.; Peng, Z.; Anne, M. V.; Nimmagadda, A.; Su, M.; Li, Y.; Sun, X.; Cai, J. Bioorg. Med. Chem. 2018, 26, 3573.
|
| [34] |
Guan, L. P.; Jin, Q. H.; Tian, G. R.; Chai, K. Y.; Quan, Z. S. J. Pharm. Pharm. Sci. 2007, 10, 254.
|
| [35] |
Pässler, U.; Knölker, H. J. Alkaloids Chem. Biol. 2011, 70, 79.
pmid: 22308756 |
| [36] |
Bhadra, K.; Kumar, G. S. Mini. Rev. Med. Chem. 2010, 10, 1235.
|
| [37] |
Jason, D. J. M.; Norman, H.; Rigdon, G. A. J. Med. Chem. 2016, 39, 149.
|
| [38] |
Kong, X. P.; Chen, Z. Z.; Xia, Y. J.; Ren, H. Q.; Dong, T. X.; Zhan, H. Q. J. Liaoning Univ. Tradit. Chin. Med. 2021, 23, 35. (in Chinese)
|
|
(孔祥鹏, 陈志从, 夏英杰, 任海琴, 董婷霞, 詹华强, 辽宁中医药大学学报, 2021, 23, 35.)
|
|
| [39] |
Guo, H. M.; Lu, Z. H.; Chen, S. Q.; Yu, Y. Asian J. Chem. 2015, 10, 3651.
|
| [40] |
Xi, M. Y.; Jia, J. M.; Sun, H. P.; Sun, Z. Y.; Jiang, J. W.; Wang, Y. J.; Zhang, M. Y. J. Med. Chem. 2013, 56, 7925.
|
| [41] |
Gan, L. L.; Gan, Z. J.; Gan, Z. J.; Dan, Y. R.; Zhang, P. M. J. Med. Chem. 2021, 64, 1018.
|
| [42] |
Grenier, D.; Audebert, S.; Preto, J.; Guichou, J. F.; Krimm, I. Expert Opin. Drug Discovery 2023, 18, 987.
|
| [43] |
Xia, M. L.; Niu, W. W.; Lin, D. Z. J. Tianjin Med. Univ. 2023, 29, 349. (in Chinese)
|
|
(夏梦琳, 牛菀菀, 林道正, 天津医科大学学报, 2023, 29, 349.)
|
|
| [44] |
Hansen, M.; Bonner, L. A.; Cueva, J. P.; Maglathlin, R.; Nichols, D. E. ACS Chem. Neurosci. 2013, 4, 96.
|
| [45] |
Ellman, G. L.; Courteny, K. D.; Andres, V.; Feather, R. M.. Biochem. Pharmacol. 1961, 7, 88.
|
| [46] |
Paulson, J. R.; Vander, E. R.; Dillinger, E.; Luedeman, M. E.; Usman, B. Chromosome Res. 2022, 30, 351.
doi: 10.1007/s10577-022-09709-1 pmid: 36399199 |
| [1] | 秦丽清, 林桂汕, 段文贵, 崔玉成, 杨卯芳, 李芳耀, 李典鹏. 新型长叶烯基萘满并N-酰基吡唑化合物的合成、抗增殖活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2024, 44(6): 1967-1977. |
| [2] | 梁国超, 董婷婷, 纪海莹, 王春艳, 宋亚丽, 张伟. 新型3,3'-((4-氯-2H-硫色烯-3-基)亚甲基)双(1H-吲哚)类拓扑异构酶Ⅱ抑制剂的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(6): 1949-1956. |
| [3] | 朱红波, 王吉, 胡炜彦, 周堂, 林芷淇, 张荣平, 耿长安, 陈兴龙. 大狼毒根茎中对胰腺癌SW1990细胞具有毒性的二萜[J]. 有机化学, 2024, 44(6): 1929-1937. |
| [4] | 裴鸿艳, 叶家麟, 王锋, 刘东东, 余裕奎, 张静, 张立新. 新型含哌啶结构的脲嘧啶类化合物的设计合成与除草活性研究[J]. 有机化学, 2024, 44(5): 1592-1605. |
| [5] | 吴思敏, 唐嘉欣, 周于佳, 徐学涛, 张昊星, 王少华. 2β-Acetoxyferruginol去醋酸基骨架衍生物抑制α-葡萄糖苷酶活性研究[J]. 有机化学, 2024, 44(2): 613-621. |
| [6] | 王锋, 陈钰, 裴鸿艳, 张静, 张立新. 含哌啶的新型1,2,4-噁二唑类衍生物的设计合成及抗真菌活性研究[J]. 有机化学, 2023, 43(8): 2826-2836. |
| [7] | 刘敏, 杨冬燕, 肖玉梅, 苏旺苍, 赵峰海, 覃兆海. 5-硝基亚氨基[1,4-2H]-1,2,4-三唑啉烯式吡虫啉类似物的合成及生物活性研究[J]. 有机化学, 2023, 43(8): 2790-2799. |
| [8] | 徐欢, 吴鸿飞, 张晓鸣, 路星星, 孙腾达, 亓悦, 林誉凡, 杨新玲, 张莉, 凌云. 含1,2,3,4-四氢异喹啉片段磺酰肼和酰肼类化合物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(2): 725-733. |
| [9] | 孙昌兴, 张福豪, 张欢, 李鹏辉, 姜林. 新型2-(1-甲基-1H-吡唑-4-基)嘧啶-4-甲酰胺的设计、合成、杀菌活性及分子对接研究[J]. 有机化学, 2023, 43(1): 229-235. |
| [10] | 李蕾, 朱聪聪, 朱全刚, 陈中建, 高希珂. 愈创木薁衍生物的设计合成及其抗氧化、抗炎活性研究[J]. 有机化学, 2022, 42(9): 2906-2913. |
| [11] | 王长凯, 孙腾达, 张学博, 杨新玲, 路星星, 徐欢, 石发胜, 张莉, 凌云. 新型含氟吡唑酰肼类化合物的设计合成与生物活性研究[J]. 有机化学, 2022, 42(5): 1527-1536. |
| [12] | 赵永梅, 穆叶舒, 罗稳, 田智勇. 胆碱酯酶抑制剂萘酰亚胺衍生物的合成与聚集诱导发光性质[J]. 有机化学, 2022, 42(3): 819-829. |
| [13] | 王秀, 段文贵, 林桂汕, 李宝谕, 张文静, 雷福厚. 含天然蒎烯结构的4-酰基-3-氨基-1,2,4-三唑-硫醚衍生物的合成、抑菌活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2022, 42(3): 871-883. |
| [14] | 孔媛芳, 杨彬, 庄严, 张京玉, 孙德梅, 董春红. 基于二肽基肽酶4 (DPP-4)靶点设计的五种降糖活性杂环合成及构效关系研究进展[J]. 有机化学, 2022, 42(3): 770-784. |
| [15] | 崔玉成, 陈美桦, 林桂汕, 段文贵, 李晴敏, 邹壬萱, 岑波. 含偕二甲基环丙烷结构的1,3,4-噻二唑-脲化合物的合成、抑菌活性及分子对接研究[J]. 有机化学, 2022, 42(11): 3784-3797. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||