有机化学 ›› 2024, Vol. 44 ›› Issue (5): 1592-1605.DOI: 10.6023/cjoc202311019 上一篇 下一篇
研究论文
裴鸿艳a,b,†, 叶家麟b,†, 王锋b, 刘东东b, 余裕奎c, 张静b,c,d,*( ), 张立新a,b,c,d,*(
), 张立新a,b,c,d,*( )
)
收稿日期:2023-11-21
修回日期:2024-01-04
发布日期:2024-01-18
作者简介:基金资助:
Hongyan Peia,b,†, Jialin Yeb,†, Feng Wangb, Dongdong Liub, Yukui Yuc, Jing Zhangb,c,d( ), Lixin Zhanga,b,c,d(
), Lixin Zhanga,b,c,d( )
)
Received:2023-11-21
Revised:2024-01-04
Published:2024-01-18
Contact:
*E-mail: About author:Supported by:文章分享

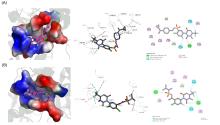
为了寻求新型高效、环境友好的除草化合物, 以2-氟-4-氯苯胺为原料, 依据活性亚结构拼接的方法, 设计合成了26个结构新颖的脲嘧啶类化合物. 其结构经1H NMR、13C NMR、HRMS分析确认. 苗后除草活性测试结果表明, 在浓度为37.5 g/hm2时, 5个化合物对阔叶杂草(百日草、苘麻)的防除效果在90%~100%; 在浓度为9.375 g/hm2时, 仍有2个化合物对阔叶杂草(百日草、苘麻)的防除效果在50%~90%, 且对禾本科杂草(稗草、金色狗尾草)无药害. 分子表面静电势研究表明, 2-氯-N-((1-(环丙烷羰基)哌啶-4-基)甲基)-4-氟-5-(3-甲基-2,6-二氧代-4-三氟甲基-3,6-二氢嘧啶- 1(2H)-基)苯磺酰胺(8i)和Saflufenacil电荷分布相近, 可能在化合物与其靶标受体对接时起重要作用. 分子对接结果表明, 化合物8i与原卟啉原氧化酶存在着多种相互作用, 以上结果为脲嘧啶类化合物的进一步优化奠定了基础.
裴鸿艳, 叶家麟, 王锋, 刘东东, 余裕奎, 张静, 张立新. 新型含哌啶结构的脲嘧啶类化合物的设计合成与除草活性研究[J]. 有机化学, 2024, 44(5): 1592-1605.
Hongyan Pei, Jialin Ye, Feng Wang, Dongdong Liu, Yukui Yu, Jing Zhang, Lixin Zhang. Design, Synthesis and Herbicidal Activity of Novel Uracil Compounds Containing Piperidine Moiety[J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1592-1605.
| Entry | Base | Solvent | Temperature | Yield/% |
|---|---|---|---|---|
| 1 | Pyridine | CH2Cl2 | r.t. | 35.9 |
| 2 | Pyridine | CH3CN | r.t. | 38.8 |
| 3 | Pyridine | Toluene | r.t. | 1.2 |
| 4 | Et3N | CH2Cl2 | r.t. | 72.8 |
| 5 | Et3N | CH3CN | r.t. | 47.7 |
| 6 | Et3N | Toluene | r.t. | 2.6 |
| 7 | DIPEA | CH2Cl2 | r.t. | 70.5 |
| 8 | DIPEA | CH3CN | r.t. | 51.7 |
| 9 | DIPEA | Toluene | r.t. | 3.9 |
| 10 | NaHCO3 | CH2Cl2 | r.t. | 53.5 |
| 11 | NaHCO3 | CH3CN | r.t. | 56.9 |
| 12 | NaHCO3 | Toluene | r.t. | 5.2 |
| 13 | Et3N | CH2Cl2 | Reflux | 63.6 |
| Entry | Base | Solvent | Temperature | Yield/% |
|---|---|---|---|---|
| 1 | Pyridine | CH2Cl2 | r.t. | 35.9 |
| 2 | Pyridine | CH3CN | r.t. | 38.8 |
| 3 | Pyridine | Toluene | r.t. | 1.2 |
| 4 | Et3N | CH2Cl2 | r.t. | 72.8 |
| 5 | Et3N | CH3CN | r.t. | 47.7 |
| 6 | Et3N | Toluene | r.t. | 2.6 |
| 7 | DIPEA | CH2Cl2 | r.t. | 70.5 |
| 8 | DIPEA | CH3CN | r.t. | 51.7 |
| 9 | DIPEA | Toluene | r.t. | 3.9 |
| 10 | NaHCO3 | CH2Cl2 | r.t. | 53.5 |
| 11 | NaHCO3 | CH3CN | r.t. | 56.9 |
| 12 | NaHCO3 | Toluene | r.t. | 5.2 |
| 13 | Et3N | CH2Cl2 | Reflux | 63.6 |
| Compd. | 百日草(ZE) | 苘麻(AT) | 金色狗尾草(SG) | 稗草(EC) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 150 g/hm2 | 37.5 g/hm2 | 150 g/hm2 | 37.5 g/hm2 | 150 g/hm2 | 37.5 g/hm2 | 150 g/hm2 | 37.5 g/hm2 | ||||
| 8a | 100 | 100 | 90 | 90 | 80 | 20 | 60 | 20 | |||
| 8b | 30 | 20 | 80 | 50 | 0 | 0 | 0 | 0 | |||
| 8c | 95 | 40 | 98 | 60 | 0 | 0 | 0 | 0 | |||
| 8d | 90 | 40 | 80 | 50 | 0 | 0 | 0 | 0 | |||
| 8e | 10 | 10 | 20 | 0 | 0 | 0 | 0 | 0 | |||
| 8f | 100 | 70 | 100 | 70 | 0 | 0 | 0 | 0 | |||
| 8g | 90 | 70 | 100 | 30 | 0 | 0 | 0 | 0 | |||
| 8h | 95 | 40 | 95 | 40 | 0 | 0 | 0 | 0 | |||
| 8i | 100 | 100 | 100 | 100 | 80 | 20 | 30 | 20 | |||
| 8j | 10 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | |||
| 8k | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 8l | 100 | 50 | 100 | 50 | 0 | 0 | 0 | 0 | |||
| 8m | 80 | 40 | 85 | 30 | 0 | 0 | 0 | 0 | |||
| 8n | 70 | 20 | 80 | 25 | 0 | 0 | 0 | 0 | |||
| 8o | 100 | 80 | 80 | 60 | 0 | 0 | 0 | 0 | |||
| 8p | 95 | 50 | 100 | 90 | 0 | 0 | 0 | 0 | |||
| 8q | 100 | 50 | 100 | 90 | 0 | 0 | 0 | 0 | |||
| 8r | 90 | 70 | 95 | 80 | 0 | 0 | 0 | 0 | |||
| 8s | 50 | 10 | 30 | 10 | 0 | 0 | 0 | 0 | |||
| 8t | 90 | 30 | 90 | 20 | 0 | 0 | 0 | 0 | |||
| 8u | 80 | 20 | 70 | 20 | 0 | 0 | 0 | 0 | |||
| 8v | 30 | 20 | 30 | 20 | 0 | 0 | 0 | 0 | |||
| 8w | 20 | 10 | 30 | 20 | 0 | 0 | 0 | 0 | |||
| 8x | 20 | 10 | 20 | 10 | 0 | 0 | 0 | 0 | |||
| 8y | 50 | 20 | 80 | 30 | 0 | 0 | 0 | 0 | |||
| 8z | 20 | 10 | 20 | 10 | 0 | 0 | 0 | 0 | |||
| Fomesafen | 100 | 90 | 40 | 20 | 0 | 0 | 0 | 0 | |||
| Saflufenacil | 100 | 100 | 100 | 100 | 100 | 80 | 80 | 50 | |||
| Compd. | 百日草(ZE) | 苘麻(AT) | 金色狗尾草(SG) | 稗草(EC) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 150 g/hm2 | 37.5 g/hm2 | 150 g/hm2 | 37.5 g/hm2 | 150 g/hm2 | 37.5 g/hm2 | 150 g/hm2 | 37.5 g/hm2 | ||||
| 8a | 100 | 100 | 90 | 90 | 80 | 20 | 60 | 20 | |||
| 8b | 30 | 20 | 80 | 50 | 0 | 0 | 0 | 0 | |||
| 8c | 95 | 40 | 98 | 60 | 0 | 0 | 0 | 0 | |||
| 8d | 90 | 40 | 80 | 50 | 0 | 0 | 0 | 0 | |||
| 8e | 10 | 10 | 20 | 0 | 0 | 0 | 0 | 0 | |||
| 8f | 100 | 70 | 100 | 70 | 0 | 0 | 0 | 0 | |||
| 8g | 90 | 70 | 100 | 30 | 0 | 0 | 0 | 0 | |||
| 8h | 95 | 40 | 95 | 40 | 0 | 0 | 0 | 0 | |||
| 8i | 100 | 100 | 100 | 100 | 80 | 20 | 30 | 20 | |||
| 8j | 10 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | |||
| 8k | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| 8l | 100 | 50 | 100 | 50 | 0 | 0 | 0 | 0 | |||
| 8m | 80 | 40 | 85 | 30 | 0 | 0 | 0 | 0 | |||
| 8n | 70 | 20 | 80 | 25 | 0 | 0 | 0 | 0 | |||
| 8o | 100 | 80 | 80 | 60 | 0 | 0 | 0 | 0 | |||
| 8p | 95 | 50 | 100 | 90 | 0 | 0 | 0 | 0 | |||
| 8q | 100 | 50 | 100 | 90 | 0 | 0 | 0 | 0 | |||
| 8r | 90 | 70 | 95 | 80 | 0 | 0 | 0 | 0 | |||
| 8s | 50 | 10 | 30 | 10 | 0 | 0 | 0 | 0 | |||
| 8t | 90 | 30 | 90 | 20 | 0 | 0 | 0 | 0 | |||
| 8u | 80 | 20 | 70 | 20 | 0 | 0 | 0 | 0 | |||
| 8v | 30 | 20 | 30 | 20 | 0 | 0 | 0 | 0 | |||
| 8w | 20 | 10 | 30 | 20 | 0 | 0 | 0 | 0 | |||
| 8x | 20 | 10 | 20 | 10 | 0 | 0 | 0 | 0 | |||
| 8y | 50 | 20 | 80 | 30 | 0 | 0 | 0 | 0 | |||
| 8z | 20 | 10 | 20 | 10 | 0 | 0 | 0 | 0 | |||
| Fomesafen | 100 | 90 | 40 | 20 | 0 | 0 | 0 | 0 | |||
| Saflufenacil | 100 | 100 | 100 | 100 | 100 | 80 | 80 | 50 | |||
| Compd. | 百日草(ZE) | 苘麻(AT) | 金色狗尾草(SG) | 稗草(EC) |
|---|---|---|---|---|
| 8a | 80 | 60 | 0 | 0 |
| 8c | 10 | 20 | 0 | 0 |
| 8f | 10 | 20 | 0 | 0 |
| 8g | 10 | 10 | 0 | 0 |
| 8h | 10 | 20 | 0 | 0 |
| 8i | 90 | 50 | 0 | 0 |
| 8l | 20 | 20 | 0 | 0 |
| 8p | 30 | 50 | 0 | 0 |
| 8q | 20 | 60 | 0 | 0 |
| 8r | 30 | 40 | 0 | 0 |
| Saflufenacil | 100 | 100 | 10 | 20 |
| Compd. | 百日草(ZE) | 苘麻(AT) | 金色狗尾草(SG) | 稗草(EC) |
|---|---|---|---|---|
| 8a | 80 | 60 | 0 | 0 |
| 8c | 10 | 20 | 0 | 0 |
| 8f | 10 | 20 | 0 | 0 |
| 8g | 10 | 10 | 0 | 0 |
| 8h | 10 | 20 | 0 | 0 |
| 8i | 90 | 50 | 0 | 0 |
| 8l | 20 | 20 | 0 | 0 |
| 8p | 30 | 50 | 0 | 0 |
| 8q | 20 | 60 | 0 | 0 |
| 8r | 30 | 40 | 0 | 0 |
| Saflufenacil | 100 | 100 | 10 | 20 |


| Compd. | ∆ETotal/a.u. | ∆ELUMO/a.u. | ∆EHOMO/a.u. | ∆Ea/a.u. |
|---|---|---|---|---|
| 8a | -2627.46164409 | -0.10851 | -0.24428 | 0.13577 |
| 8i | -2510.73775329 | -0.10789 | -0.23744 | 0.12955 |
| Saflufenacil | -2510.72139594 | -0.11012 | -0.26993 | 0.15981 |
| Compd. | ∆ETotal/a.u. | ∆ELUMO/a.u. | ∆EHOMO/a.u. | ∆Ea/a.u. |
|---|---|---|---|---|
| 8a | -2627.46164409 | -0.10851 | -0.24428 | 0.13577 |
| 8i | -2510.73775329 | -0.10789 | -0.23744 | 0.12955 |
| Saflufenacil | -2510.72139594 | -0.11012 | -0.26993 | 0.15981 |

| [1] |
Xun, X.; Ni, Y.-D.; Li, J.-F.; Ding, Y.; Zhang, M.; Wang, Y.; Hu, L.-P.; Zhou, H.-Y.; Yang, B.; Shi, J.; Gao, L.; Dai, H. Chin. J. Org. Chem. 2020, 40, 1638 (in Chinese).
|
|
( 荀校, 倪亚丹, 李金峰, 丁颖, 张敏, 王杨, 胡兰萍, 周环宇, 杨冰, 石健, 高磊, 戴红, 有机化学, 2020, 40, 1638.)
doi: 10.6023/cjoc202002024 |
|
| [2] |
Wang, F.; Chen, Y.; Pei, H.-Y.; Zhang, J.; Zhang, L.-X. Chin. J. Org. Chem. 2023, 43, 2826 (in Chinese).
|
|
( 王锋, 陈钰, 裴鸿艳, 张静, 张立新, 有机化学, 2023, 43, 2826.)
doi: 10.6023/cjoc202212015 |
|
| [3] |
Ma, D.; Yin, Y.; Chen, Y.-L.; Yan, Y.-T.; Wu, J. RSC Adv. 2021, 11, 15380.
|
| [4] |
Ding, C.-R.; Pan, Y.-Y.; Tan, C.-X. Chin. J. Org. Chem. 2020, 40, 528 (in Chinese).
|
|
( 丁成荣, 潘亚运, 谭成侠, 有机化学, 2020, 40, 528.)
doi: 10.6023/cjoc201907034 |
|
| [5] |
Abdelshaheed, M.-M.; Fawzy, I.-M.; El-Subbagh, H.-I.; Youssef, K.-M. Future J. Pharm. Sci. 2021, 7, 1.
|
| [6] |
Li, J.-J.; Dai, L.-L.; Huang, W.-H.; Bi, Y.-L. Chin. J. Pestic. 2019, 58, 703 (in Chinese).
|
|
( 李君君, 戴玲玲, 黄文化, 毕亚玲, 农药, 2019, 58, 703.)
|
|
| [7] |
Zhang, S.-L.; Pei, H.-Y.; Sheng, Z.-B.; Gao, Y.-X.; Zhang, J.; Zhang, L.-X. Mod. Agrochem. 2021, 20, 6 (in Chinese).
|
|
( 张石磊, 裴鸿艳, 盛祝波, 高一星, 张静, 张立新, 现代农药, 2021, 20, 6.)
|
|
| [8] |
Wang, D. W.; Lu, L.; Xue, Z. Y.; Yu, S. Y.; Zhang, R. B.; Xia, W.; Han, X.; Xin, W.; Zhen, X. J. Agric. Food Chem. 2021, 69, 4081.
|
| [9] |
Park, J.; Ahn, Y. O.; Nam, J. W.; Hong, M. K.; Song, N.; Kim, T.; Yu, G. H.; Sung, S. K. Pestic. Biochem. Physiol. 2018, 152, 38.
|
| [10] |
Yang, S.; Tang, J. H.; Li, B. J.; Yao, G. K.; Peng, H. X.; Pu, C. M.; Zhao, C.; Xu, H. H. J. Agric. Food Chem. 2023, 71, 14221-14231.
|
| [11] |
Cha, J. Y.; Lee, D. Y.; Ali, I.; Jeong, S. Y.; Shin, B.; Ji, H.; Kim, J. S.; Kim, M. G.; Kim, W. Y. Plant Cell Rep. 2019, 38, 793.
|
| [12] |
Fukunaga, S.; Ogata, K.; Eguchi, A.; Matsunaga, K.; Sakurai, K., Abe, J.; Cohen, M.-C.; Asano, H. Regul. Toxicol. Pharmacol. 2022, 136, 105268.
|
| [13] |
Ivantsova, E.; Konig, I.; Lopez-Scarim, V.; English, C.; Charnas, S.-R.; Souders, C.-L.; Martyniuk, C.-J. Environ. Toxicol. Pharmacol. 2023, 98, 104084.
|
| [14] |
Li, P. S. WO 2022095816, 2022.
|
| [15] |
Qu, R. Y.; He, B.; Yang, J. F.; Yang, W. C.; Wu, Q. Y.; Li, Q. X.; Yang, G. F. Pest Manage. Sci. 2021, 77, 2620.
|
| [16] |
Tan, H.-J. World Pestic. 2022, 44(9), 1 (in Chinese).
|
|
( 谭海军, 世界农药, 2022, 44(9), 1.)
|
|
| [17] |
Yang, S.; Tang, J. H.; Li, B. J.; Yao, G. K.; Peng, H. X.; Pu, C. M.; Zhao, C.; Xu, H. H. J. Agric. Food Chem. 2023, 71, 14221.
|
| [18] |
Yu, J. J.; Jiang X. F. Adv. Agrochem. 2023, 2, 3.
|
| [19] |
Luan, T.; Cui, S.-J.; Sui, L.-L.; Sun, C.-Y.; Zhang, D.-J. Chem. J. Chin. Univ. 2023, 44, 20230098 (in Chinese).
|
|
( 栾天, 崔思娇, 隋丽丽, 孙驰宇, 张大军, 高等学校化学学报, 2023, 44, 20230098.)
doi: 10.7503/cjcu20230098 |
|
| [20] |
Wen, Y.-P.; Zhang, S.; Hou, G.-F.; Yu, Y.-H.; Gao, J.-S. Chin. J. Org. Chem. 2016, 36, 642 (in Chinese).
|
|
( 温彦鹏, 张爽, 侯广峰, 于颖慧, 高金胜, 有机化学, 2016, 36, 642.)
doi: 10.6023/cjoc201508008 |
|
| [21] |
Li, K.-M.; Li, Y.-S.; Yi, Y.-J.; Xu, L.-T.; Ye, J.; Ou, X.-M.; Li, J.-M.; Hu, A.-X. Chem. J. Chin. Univ. 2020, 41, 716 (in Chinese).
|
|
( 李康明, 李延赛, 易阳杰, 徐雷涛, 叶姣, 欧晓明, 李建明, 胡艾希, 高等学校化学学报, 2020, 41, 716.)
doi: 10.7503/cjcu20190504 |
|
| [22] |
Singh, I.; Al-Wahaibi, L. H.; Srivastava, R.; Prasad, O.; Pathak, S. K.; Kumar, S.; Parveen, S.; Banerjee, M.; El-Emam, A. A.; Sinha, L. ACS Omega 2020, 5, 46.
|
| [23] |
Yang, J. C.; Wu, E. M.; Jin, S. Z.; Wu, J.; Ying, J. W.; Yang, F.; Liu, C. L. CN 108570041, 2018.
|
| [24] |
Lee, K.; Kuk, J.; Ahn, Y.; Lee, J. WO 2018128387, 2018.
|
| [25] |
Liu, A.-C.; Fang, Q.; Wu, Z. H.; Xu, Y.-L.; Chen, D.-F. World Pestic. 2023, 45(09), 19 (in Chinese).
|
|
( 刘安昌, 方强, 吴子豪, 徐婴兰, 陈典富, 世界农药, 2023, 45(09), 19.)
|
|
| [26] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V..; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H.; Pizmaylov, A.; Fbloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J.J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, N. J.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J.J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, revision D.01, Gaussian, Inc., Wallingford, CT, 2013.
|
| [27] |
Chen, W.; Lei, S.-M.; Lan, Y.-X.; Xu, H.-J.; Yu, P.-B.; Zhang, R.; Wu, R.; Chen, Y. Chin. J. Org. Chem. 2022, 42, 2164 (in Chinese).
|
|
( 陈伟, 雷思敏, 兰雨欣, 许豪键, 余坪槟, 张锐, 吴润, 陈阳, 有机化学, 2022, 42, 2164.)
doi: 10.6023/cjoc202201020 |
|
| [28] |
Ibrahim, S.; Ghabi, A.; Amiri, N.; Mtiraoui, H.; Hajji, M.; Bel-Hadj-Tahar, R.; Msaddek, M. Monatsh. Chem. 2021, 152, 523.
|
| [29] |
Koch, M.; Breithaupt, C.; Kiefersauer, R.; Freigang, J.; Huber, R.; Messerschmidt, A. EMBO J. 2004, 23, 1720.
|
| [30] |
Gao, W.; Li, X.; Ren, D.; Sun, S.; Huo, J.; Wang, Y.; Chen, L.; Zhang, J. Molecules 2019, 24, 4363.
|
| [1] | 李章健, 王振华, 郭剑峰, 方萍, 马聪, 刘润华, 梅天胜. 电化学促进2,2,6,6-四甲基哌啶氧化物(TEMPO)介导的甘氨酸衍生物氧化脱氢Povarov/串联反应[J]. 有机化学, 2024, 44(3): 940-950. |
| [2] | 吴思敏, 唐嘉欣, 周于佳, 徐学涛, 张昊星, 王少华. 2β-Acetoxyferruginol去醋酸基骨架衍生物抑制α-葡萄糖苷酶活性研究[J]. 有机化学, 2024, 44(2): 613-621. |
| [3] | 王锋, 陈钰, 裴鸿艳, 张静, 张立新. 含哌啶的新型1,2,4-噁二唑类衍生物的设计合成及抗真菌活性研究[J]. 有机化学, 2023, 43(8): 2826-2836. |
| [4] | 刘敏, 杨冬燕, 肖玉梅, 苏旺苍, 赵峰海, 覃兆海. 5-硝基亚氨基[1,4-2H]-1,2,4-三唑啉烯式吡虫啉类似物的合成及生物活性研究[J]. 有机化学, 2023, 43(8): 2790-2799. |
| [5] | 林海, 聂会祥, 赵安林, 王涛, 罗劲. 新型嘧啶并[5,4-e][1,2,4]三唑并[1,5-c]嘧啶衍生物的合成与除草活性[J]. 有机化学, 2023, 43(7): 2462-2475. |
| [6] | 徐欢, 吴鸿飞, 张晓鸣, 路星星, 孙腾达, 亓悦, 林誉凡, 杨新玲, 张莉, 凌云. 含1,2,3,4-四氢异喹啉片段磺酰肼和酰肼类化合物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(2): 725-733. |
| [7] | 汪蕾, 于淑晶, 杨娜, 王宝雷. 新型含二氢喹唑啉酮的咖啡因衍生物的合成及生物活性研究[J]. 有机化学, 2023, 43(1): 299-307. |
| [8] | 孙昌兴, 张福豪, 张欢, 李鹏辉, 姜林. 新型2-(1-甲基-1H-吡唑-4-基)嘧啶-4-甲酰胺的设计、合成、杀菌活性及分子对接研究[J]. 有机化学, 2023, 43(1): 229-235. |
| [9] | 陈伟, 雷思敏, 兰雨欣, 许豪键, 余坪槟, 张锐, 吴润, 陈阳. 新型喹唑啉酮衍生物的设计合成与抗植物病原真菌活性研究[J]. 有机化学, 2022, 42(7): 2164-2171. |
| [10] | 王长凯, 孙腾达, 张学博, 杨新玲, 路星星, 徐欢, 石发胜, 张莉, 凌云. 新型含氟吡唑酰肼类化合物的设计合成与生物活性研究[J]. 有机化学, 2022, 42(5): 1527-1536. |
| [11] | 王秀, 段文贵, 林桂汕, 李宝谕, 张文静, 雷福厚. 含天然蒎烯结构的4-酰基-3-氨基-1,2,4-三唑-硫醚衍生物的合成、抑菌活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2022, 42(3): 871-883. |
| [12] | 孔媛芳, 杨彬, 庄严, 张京玉, 孙德梅, 董春红. 基于二肽基肽酶4 (DPP-4)靶点设计的五种降糖活性杂环合成及构效关系研究进展[J]. 有机化学, 2022, 42(3): 770-784. |
| [13] | 崔玉成, 陈美桦, 林桂汕, 段文贵, 李晴敏, 邹壬萱, 岑波. 含偕二甲基环丙烷结构的1,3,4-噻二唑-脲化合物的合成、抑菌活性及分子对接研究[J]. 有机化学, 2022, 42(11): 3784-3797. |
| [14] | 陈睿嘉, 周聪, 逄锡文, 刘佳君, 顾玉诚, 刘建文, 李忠. 以环丙烷限制构象的新型双酰胺的设计、合成、抗癌活性及计算分析[J]. 有机化学, 2022, 42(1): 277-292. |
| [15] | 李英俊, 林乐弟, 刘季红, 高立信, 盛丽, 靳焜, 刘雪洁, 杨鸿境, 李佳. 新型含咔唑环和芳环/芳稠杂环的N-酰腙衍生物的合成及蛋白酪氨酸磷酸酶1B (PTP1B)抑制活性评价[J]. 有机化学, 2021, 41(9): 3593-3607. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||