有机化学 ›› 2024, Vol. 44 ›› Issue (7): 2341-2349.DOI: 10.6023/cjoc202401012 上一篇 下一篇
研究论文
徐童a, 张宁a, 张永红a, 王斌a, 夏昱a, 金伟伟b,*( ), 金聘入c,*(
), 金聘入c,*( ), 刘晨江a,*(
), 刘晨江a,*( )
)
收稿日期:2024-01-12
修回日期:2024-03-07
发布日期:2024-03-28
基金资助:
Tong Xua, Ning Zhanga, Yonghong Zhanga, Bin Wanga, Yu Xiaa, Weiwei Jinb( ), Pinru Jinc(
), Pinru Jinc( ), Chenjiang Liua(
), Chenjiang Liua( )
)
Received:2024-01-12
Revised:2024-03-07
Published:2024-03-28
Contact:
E-mail: Supported by:文章分享
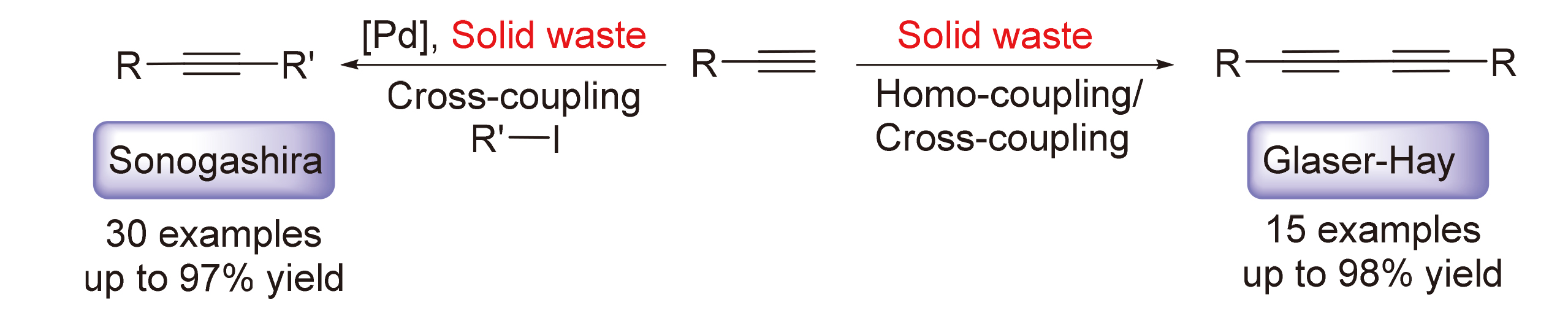
开发了一种使用铜基固体废弃物代替传统铜盐参与Sonogashira和Glaser-Hay偶联反应的方法, 在较为温和的条件下, 高效地合成了多种二取代炔烃. 对于Sonogashira偶联反应, 各种取代的苯乙炔和碘代芳烃反应效果良好, 以57%~97%的产率生成二芳基乙炔类化合物. 对于Glaser-Hay偶联反应, 多种末端炔烃既能以优秀的产率生成对称二取代1,3-丁二炔衍生物, 也能以中等的产率合成交叉偶联产物. 克级规模实验和循环实验表明该固体废弃物在有机合成中有潜在的应用前景.
徐童, 张宁, 张永红, 王斌, 夏昱, 金伟伟, 金聘入, 刘晨江. 铜基固体废弃物促进的末端炔烃交叉偶联反应[J]. 有机化学, 2024, 44(7): 2341-2349.
Tong Xu, Ning Zhang, Yonghong Zhang, Bin Wang, Yu Xia, Weiwei Jin, Pinru Jin, Chenjiang Liu. Copper-Based Solid Wastes Promoted Cross-Coupling Reactions of Terminal Alkynes[J]. Chinese Journal of Organic Chemistry, 2024, 44(7): 2341-2349.
| Entry | Pd catalyst | Solid waste/mg | Base | Solvent | 1a∶2a | T/℃ | Atmosphere | Yieldb/% |
|---|---|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | 200 | Et3N | DMSO | 1∶2 | 60 | N2 | 66 |
| 2 | PdCl2 | 200 | Et3N | DMSO | 1∶2 | 60 | N2 | 76 |
| 3 | Pd(PPh3)4 | 200 | Et3N | DMSO | 1∶2 | 60 | N2 | 93 |
| 4 | Pd(PPh3)4 | 200 | Et3N | H2O | 1∶2 | 60 | N2 | 32 |
| 5 | Pd(PPh3)4 | 200 | Et3N | EtOH | 1∶2 | 60 | N2 | 39 |
| 6 | Pd(PPh3)4 | 200 | Et3N | EG | 1∶2 | 60 | N2 | 60 |
| 7 | Pd(PPh3)4 | 200 | Et3N | PEG-400 | 1∶2 | 60 | N2 | 64 |
| 8 | Pd(PPh3)4 | 200 | Et3N | PEG-400 | 2∶1 | 60 | N2 | 90 |
| 9 | Pd(PPh3)4 | 200 | Et3N | PEG-400 | 2∶1 | 30 | N2 | 15 |
| 10 | Pd(PPh3)4 | 200 | Et3N | PEG-400 | 2∶1 | 60 | Air | 96 |
| 11 | — | 200 | Et3N | PEG-400 | 2∶1 | 60 | Air | N.R. |
| 12 | Pd(PPh3)4 | 0 | Et3N | PEG-400 | 2∶1 | 60 | Air | 60 |
| 13 | Pd(PPh3)4 | 200 | — | PEG-400 | 2∶1 | 60 | Air | 16 |
| Entry | Pd catalyst | Solid waste/mg | Base | Solvent | 1a∶2a | T/℃ | Atmosphere | Yieldb/% |
|---|---|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | 200 | Et3N | DMSO | 1∶2 | 60 | N2 | 66 |
| 2 | PdCl2 | 200 | Et3N | DMSO | 1∶2 | 60 | N2 | 76 |
| 3 | Pd(PPh3)4 | 200 | Et3N | DMSO | 1∶2 | 60 | N2 | 93 |
| 4 | Pd(PPh3)4 | 200 | Et3N | H2O | 1∶2 | 60 | N2 | 32 |
| 5 | Pd(PPh3)4 | 200 | Et3N | EtOH | 1∶2 | 60 | N2 | 39 |
| 6 | Pd(PPh3)4 | 200 | Et3N | EG | 1∶2 | 60 | N2 | 60 |
| 7 | Pd(PPh3)4 | 200 | Et3N | PEG-400 | 1∶2 | 60 | N2 | 64 |
| 8 | Pd(PPh3)4 | 200 | Et3N | PEG-400 | 2∶1 | 60 | N2 | 90 |
| 9 | Pd(PPh3)4 | 200 | Et3N | PEG-400 | 2∶1 | 30 | N2 | 15 |
| 10 | Pd(PPh3)4 | 200 | Et3N | PEG-400 | 2∶1 | 60 | Air | 96 |
| 11 | — | 200 | Et3N | PEG-400 | 2∶1 | 60 | Air | N.R. |
| 12 | Pd(PPh3)4 | 0 | Et3N | PEG-400 | 2∶1 | 60 | Air | 60 |
| 13 | Pd(PPh3)4 | 200 | — | PEG-400 | 2∶1 | 60 | Air | 16 |
| Entry | Variation from the standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 98 |
| 2 | DEEDA instead of TMEDA | N.R. |
| 3 | DMEDA instead of TMEDA | N.R. |
| 4 | 20 mol% Et3N instead of TMEDA | 40 |
| 5 | 40 mol% Et3N instead of TMEDA | Trace |
| 6 | EtOH instead of CH3CN | 69 |
| 7 | PEG-400 instead of CH3CN | 57 |
| 8 | Without solid Waste | Trace |
| 9 | Without TMEDA | Trace |
| Entry | Variation from the standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 98 |
| 2 | DEEDA instead of TMEDA | N.R. |
| 3 | DMEDA instead of TMEDA | N.R. |
| 4 | 20 mol% Et3N instead of TMEDA | 40 |
| 5 | 40 mol% Et3N instead of TMEDA | Trace |
| 6 | EtOH instead of CH3CN | 69 |
| 7 | PEG-400 instead of CH3CN | 57 |
| 8 | Without solid Waste | Trace |
| 9 | Without TMEDA | Trace |


| Entry | Cu content of solid waste/(μg•g–1) |
|---|---|
| Before reaction | 50253 |
| After reaction | 5173 |
| Entry | Cu content of solid waste/(μg•g–1) |
|---|---|
| Before reaction | 50253 |
| After reaction | 5173 |
| [1] |
(a) Chinchilla, R.; Carmen Najera, C. Chem. Rev. 2014, 114, 1783.
doi: 10.1021/cr400133p pmid: 23789922 |
|
(b) Stang, P. J.; Diederich, F. Modern Acetylene Chemistry, VCH, Weinheim, 1995.
pmid: 23789922 |
|
|
(c) Diederich, F.; Stang, P. J. Acetylene Chemistry, Ed.: Tykwinski, R. R., Wiley-VCH, Weinheim, 2005.
pmid: 23789922 |
|
|
(d) Feng, J.; Zhang, F; Shu, C.-Y.; Zhu, G.-G. Chin. J. Chem. 2022, 40, 1667.
pmid: 23789922 |
|
|
(e) Hao, T.-G.; Shi, M.; Wei, Y. Chin. J. Chem. 2023, 41, 301.
pmid: 23789922 |
|
|
(f) Batchu, V. R.; Subramanian, V.; Parasuraman, K.; Swamy, N. K.; Kumar, S.; Pal, M. Tetrahedron 2005, 61, 9869.
pmid: 23789922 |
|
| [2] |
(a) Armstrong, K. M.; Lalic, G. J. Am. Chem. Soc. 2019, 141, 6173.
doi: 10.1021/jacs.9b02372 pmid: 30942593 |
|
(b) Hamasaka, G.; Roy, D.; Tazawa, A.; Uozumi, Y. ACS Catal. 2019, 9, 11640.
doi: 10.1021/acscatal.9b04593 pmid: 30942593 |
|
|
(c) Liu, L.; Dan Zhou, D.; Liu, M.; Zhou, Y.-B.; Chen, T.-Q. Org. Lett. 2018, 20, 2741.
pmid: 30942593 |
|
|
(d) Tian, Z.-Y.; Wang, S.-M.; Jia, S.-J.; Song, H.-X.; Zhang, C.-P. Org. Lett. 2017, 19, 5454.
pmid: 30942593 |
|
|
(e) Liu, B.-Q.; Yan, Z.-F.; Quan, Z.-J. Chin. J. Org. Chem. 2018, 38, 3032. (in Chinese)
pmid: 30942593 |
|
|
(刘伯渠, 燕中飞, 权正军, 有机化学, 2018, 38, 3032.)
doi: 10.6023/cjoc201803025 pmid: 30942593 |
|
| [3] |
(a) Ma, N.; Zeng, X.-H. Chin. J. Org. Chem. 2018, 38, 1556. (in Chinese)
pmid: 29450996 |
|
(马楠, 曾祥华, 有机化学, 2018, 38, 1556.)
doi: 10.6023/cjoc201712038 pmid: 29450996 |
|
|
(b) Li, X.-W.; Liu, X.-H.; Chen, H.-J.; Wu, W.-Q.; Qi, C.-F.; Jiang, H.-F. Angew. Chem., Int. Ed. 2014, 53, 14485.
pmid: 29450996 |
|
|
(c) Sakamoto, R.; Kato, T.; Sakurai, S.; Maruoka, K. Org. Lett. 2018, 20, 1400.
doi: 10.1021/acs.orglett.8b00173 pmid: 29450996 |
|
|
(d) Feng, L.-L.; Hu, T.-J.; Zhang, S.-S.; Xiong, H.-Y.; Zhang, G.-W. Org. Lett. 2019, 21, 9487.
pmid: 29450996 |
|
|
(e) Lv, Y.-H.; Pu, W.-Y.; Shi, L.-H. Org. Lett. 2019, 21, 6034.
pmid: 29450996 |
|
|
(f) Biswas, S.; Mullick, K.; Chen, S.-Y.; Kriz, D. A.; Shakil, M.; Kuo, C.-H.; Angeles-Boza, A. M.; Rossi, A. R.; Suib, S. L. ACS Catal. 2016, 6, 5069.
pmid: 29450996 |
|
| [4] |
(a) Zhang, G.; Shao, X.-B.; Li, Q.-H.; Yang, X.-J. Chin. J. Org. Chem. 2018, 38, 1538. (in Chinese)
|
|
(张刚, 杓学蓓, 李清寒, 杨学军, 有机化学, 2018, 38, 1538.)
doi: 10.6023/cjoc201802019 |
|
|
(b) Chen, H.; Yao, L.-C.; Guo, L.; Liu, Y. A.; Tian, B.-X.; Liao, X.-B. Cell Rep. Phys. Sci. 2023, 4, 101573.
|
|
|
(c) Li, Y.-Q.; Li, F.; Shi, S.-L. Chin. J. Chem. 2020, 38, 1035.
|
|
| [5] |
Islam, K. M. N. Renewable Sustainable Energy Rev. 2018, 81, 2472.
|
| [6] |
Martins, M. A. D. B.; Crispim, A.; Ferreira, M. L.; Dos Santos, I. F.; Melo, M. D. L. N.; Barros, R. M.; Filho, G. L. T. Cleaner Waste Systems 2023, 4, 100070
|
| [7] |
Ding, Y.; Zhao, J.; Liu, J.; Zhou, J.; Cheng, L.; Zhao, J.; Shao, Z.; Iris, Ç.; Pan, B.; Li, X.; Hu, Z. J. Clean. Prod. 2021, 293, 126144.
|
| [8] |
(a) Yu, H.-X.; Zahidi, I.; Liang, D.-F. J. Mater. Res. Technol. 2023, 23, 5733.
|
|
(b) Arenas, C.; Ríos, D. J.; Cifuentes, H.; Vilches, F. L.; Leiva, C. Eur. J. Environ. Civ. Eng. 2022, 9, 3805.
|
|
|
(c) Zhang, F.; Yu, W.; Liu, W.-Y.; Xu, Z.-Y. Front. Energy Res. 2020, 8, 50.
|
|
|
(d) Liu, B.-C.; Han, B.-R.; Liang, X.-Q.; Liu, Y.-F. Int. J. Hydrogen Energy 2024, 52, 1445.
|
|
| [9] |
(a) Dewan, A.; Sarmah, M.; Bora, U.; Thakur, A. J. Tetrahedron Lett. 2016, 57, 3760.
|
|
(b) Dewan, A.; Sarmah, M.; Bora, U.; Thakur, A. J. Appl. Organomet. Chem. 2017, 31, e3646.
|
|
|
(c) Dewan, A.; armah, M.; Thakur, A. J.; Bharali, P.; Bora, U. ACS Omega 2018, 3, 5327.
|
|
|
(d) Boruah, P.-R.; Ali, A.-A.; Saikia, B.; Sarma, D. Green Chem. 2015, 17, 1442.
|
|
| [10] |
(a) Isfahani, A. L.; Mohammadpoor-Baltork, I.; Mirkhani, V.; hosropour, A. R.; Moghadam, M.; Tangestaninejad, S. Eur. J. Org. Chem. 2014, 5603.
|
|
(b) Thathagar, M. B.; Beckers, J.; Rothenberg, G. Green Chem. 2004, 6, 215.
|
|
|
(c) Zhang, C.-T.; Peng, L.-J.; Song, B.-H.; Li, Z.-W.; Cao, X.-Q. Inorg. Chem. Commun. 2023, 158, 111471.
|
|
|
(d) Moghaddam, F. M.; Tavakoli, G.; Rezvani, H. R. Catal. Commun. 2015, 60, 82.
|
|
|
(e) Mohammadi, P.; Heravil, M. M.; Mohammadi, L.; Saljooqi, A. Sci. Rep. 2023, 13, 17375.
|
|
| [11] |
(a) Tang, S.-Y.; Li, L.-J.; Ren, X.-H.; Li, J.; Yang, G.-Y.; Li, H.; Yuan, B.-X. Green Chem. 2019, 21, 2899.
|
|
(b) Su, L.-B.; Dong, J.-Y.; Liu, L.; Sun, M.-L.; Qiu, R.-H.; Zhou, Y.-B.; Yin, S.-F. J. Am. Chem. Soc. 2016, 138, 12348.
|
|
|
(c) Kusuda, A.; Xu, X.-X.; Wang, X.; Tokunaga, E.; Shibata, N. Green Chem. 2011, 13, 843.
|
|
|
(d) Zhang, L.-Z.; Wei, C.-B.; Wu, J.-W.; Liu, D.; Yao, Y.-C.; Chen, Z.; Liu, J.-X.; Yao, C.-J.; Li, D.-H.; Yang, R.-J.; Xia, Z.-H. Chem. Sci. 2022, 13, 7475.
|
|
| [12] |
(a) Sun, Y.-J.; Wang, R.; Liu, T.-X.; Jin, W.-W.; Wang, B.; Zhang, Y.-H.; Xia, Y.; Liu, C.-J. Eur. J. Org. Chem. 2021, 2470.
|
|
(b) Sun, Y.-J.; Jin, W.-W.; Liu, C.-J. Molecules 2019, 24, 3838.
|
|
| [13] |
Denis, P.; Andy, W.; John, H.; Sneddon, H.; C. Robert, M.; Sarah, A. S.; Peter J. D. Green Chem. 2016, 18, 288.
|
| [14] |
Zhao, C.-Q.; Chen, Y.-G.; Qiu, H.; Wei, L.; Fang, P.; Mei, T.-S. Org. Lett. 2019, 21, 1412.
|
| [15] |
Stein, A.-L.; Bilheria, F.-N.; Zeni, G. Chem. Commun. 2015, 51, 15522.
|
| [16] |
(a) Qiu, S.-Z.; Zhang, C.-Y.; Qiu, R.; Yin, G.-D.; Huang, J.-K. Adv. Synth. Catal. 2018, 360, 313.
pmid: 15651824 |
|
(b) Yu, S.-Y.; Wu, J.-X.; He, X.-W.; Shang, Y.-J. Appl. Organomet. Chem. 2018, 32, e4156.
pmid: 15651824 |
|
|
(c) Topolovčan, N.; Hara, S.; Císařová, I.; Tošner, Z.; Kotora, M. Eur. J. Org. Chem. 2020, 2, 234.
pmid: 15651824 |
|
|
(d) Jitendra, R. H.; Theodore, J. A.; Alwyn, T. G.; Garcia, M. T.; Robert, D. S.; Peter, J. S. Green Chem. 2010, 12, 650.
pmid: 15651824 |
|
|
(e) Pati, A. K.; Mohapatra, M.; Ghosh, P.; Gharpure, S. J.; Mishra, A. K. J. Phys. Chem. A 2013, 117, 6548.
pmid: 15651824 |
|
|
(f) Kurita, T.; Abe, M.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Synlett 2007, 16, 2521.
pmid: 15651824 |
|
|
(g) Chen, S.-N.; Wu, W.-Y.; Tsai, F.-Y. Green Chem. 2009, 11, 269.
pmid: 15651824 |
|
|
(h) Batsanov, A. S.; Collings, J. C.; Fairlamb, I. J. S.; Holland, J. P.; Howard, J. A. K.; Lin, Z.-Y.; Marder, T. B.; Parsons, A. C.; Ward, R. M.; Zhu, J. J. Org. Chem. 2005, 70, 703.
pmid: 15651824 |
|
|
(i) Li, X.; Li, D.-J.; Bai, Y.-N.; Zhang, C.-X.; Chang, H.-H.; Gao, W.-C.; Wei, W.-L. Tetrahedron 2016, 72, 6996.
pmid: 15651824 |
|
|
(j) Zhang, S.-L.; Liu, X.-Y.; Wang, T.-Q. Adv. Synth. Catal. 2011, 353, 1463.
pmid: 15651824 |
|
|
(k) Chinta, B. S.; Baire, B. RSC Adv. 2016, 6, 54449.
pmid: 15651824 |
| [1] | 王银银, 林晓婉, 张飘, 沈美华, 徐华栋, 徐德锋. 基于吡啶和1,3,5-三嗪的PNP钳状配体的设计合成以及在钴催化的末端炔烃半氢化反应中的应用[J]. 有机化学, 2021, 41(8): 3312-3320. |
| [2] | 胡志芳, 彭丽芬, 邱仁华, 折田明浩. 末端炔烃保护基的研究进展[J]. 有机化学, 2020, 40(10): 3112-3119. |
| [3] | 孔胜男, Abaid Ullah Malik, 钱雪峰, 舒谋海, 肖文德. 水相中微孔有机聚合物负载钯催化的碳-碳偶联反应[J]. 有机化学, 2018, 38(2): 432-442. |
| [4] | 彭丽芬, 彭超, 汪明, 唐子龙, 焦银春, 许新华. 膦酰保护基促进选择性Hay偶联制备非对称1,3-二炔[J]. 有机化学, 2018, 38(11): 3048-3055. |
| [5] | 戚海棠, 宋光琳, 权正军, 王喜存. CuSO4·5H2O/NaAsc催化下碘代芳烃和末端炔烃的Sonogashira偶联反应[J]. 有机化学, 2017, 37(7): 1855-1859. |
| [6] | 白亚丽, 李晓维, 肖雪冬, 刘佳琦, 杨俊娟, 王君文. 含羟基氮杂环卡宾的咪唑盐前体的合成及其原位产生的卡宾配体在Suzuki-Miyaura和Sonogashira反应中的应用[J]. 有机化学, 2017, 37(5): 1258-1265. |
| [7] | 关志朋, 师瑶, 石炜, 陈浩. 温和条件下去丙酮保护制备末端单炔烃及末端二炔的方法[J]. 有机化学, 2017, 37(2): 418-422. |
| [8] | 袁航, 陈惠莲, 罗治斌, 高玉华, 陆鸿飞. 一种含吡啶配体氮杂环卡宾钯络合物(NHC)PdCl2(Py)的合成及其高效催化偶联反应[J]. 有机化学, 2017, 37(11): 2948-2955. |
| [9] | 李福卫, 王小龙, 于海涛. 铜催化腙酰卤与端炔的串联反应制备1,3,5-三取代吡唑衍生物[J]. 有机化学, 2016, 36(5): 1127-1132. |
| [10] | 李亦彪, 程亮, 陈路, 李滨, 孙宁, 卿宁. 基于末端炔烃一锅法合成取代噻吩和呋喃化合物[J]. 有机化学, 2016, 36(10): 2426-2436. |
| [11] | 纪宪勇, 张婧, 汤灿林, 王杰. 间苯二甲酸酯为端基的非对称共轭树状体的合成及光学性质[J]. 有机化学, 2011, 31(01): 126-131. |
| [12] | 王 翔a,b; 赖媛媛a,c ; 吴 宏a ; 张建明a ; 李永建*,a. 金催化的吲哚与末端炔烃的分子间烷基化反应[J]. 有机化学, 2009, 29(03): 432-436. |
| [13] | 陶李明,梁云,李金恒a. 末端炔烃二聚反应合成共轭烯炔的研究进展[J]. 有机化学, 2007, 27(9): 1078-1086. |
| [14] | 王阿忠,江焕峰. 超临界二氧化碳中PdCl2-CuCl2体系催化末端炔烃氧化偶联反应[J]. 有机化学, 2007, 27(05): 619-622. |
| [15] | 石启英. Pd(PPh3)2Cl2-CuCl体系催化有机高价碘杂环化合物与末端炔烃的化学选择性交叉偶联反应[J]. 有机化学, 2004, 24(8): 912-915. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||