有机化学 ›› 2025, Vol. 45 ›› Issue (4): 1369-1378.DOI: 10.6023/cjoc202406026 上一篇 下一篇
研究论文
收稿日期:2024-06-20
修回日期:2024-09-15
发布日期:2024-10-10
基金资助:
Mingmei Zhang, Feng Sha, Xin-Yan Wu( )
)
Received:2024-06-20
Revised:2024-09-15
Published:2024-10-10
Contact:
* E-mail: Supported by:文章分享
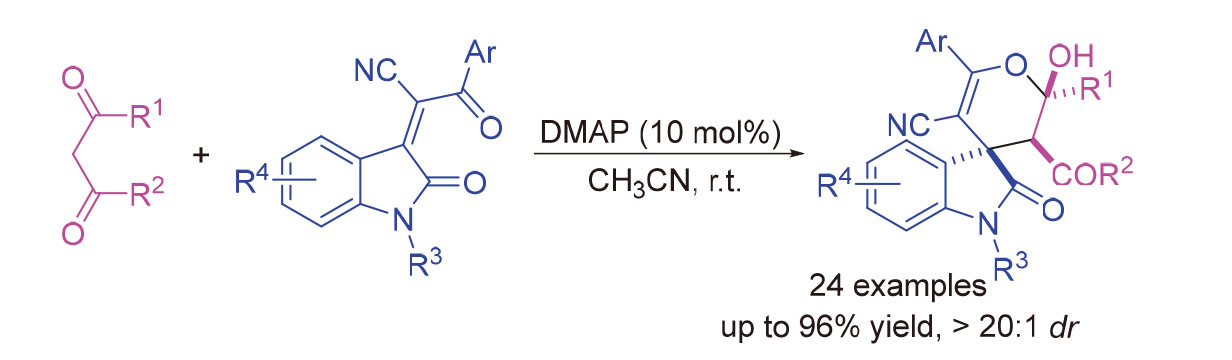
3,4'-吡喃-螺环氧化吲哚结构单元存在于一些天然产物和具有生物活性的合成化合物中, 而多官能团化3,4'-吡喃-螺环氧化吲哚的合成鲜有文献报道. 本工作发展了一种4-二甲氨基吡啶(DMAP)催化作用下β-二羰基化合物与靛红衍生的α,β-不饱和酮之间的Michael加成/半缩酮化串联反应, 在温和条件下, 以最高99%的产率和大于20∶1的非对映选择性成功地合成了一系列多官能团化3,4'-吡喃-螺环氧化吲哚类化合物. 该研究为高效构建多官能团化的3,4'-吡喃-螺环氧吲哚类化合物提供了一个简单、温和的方法.
张明美, 沙风, 伍新燕. β-二羰基化合物与靛红衍生的α,β-不饱和酮之间的Michael加成/半缩酮化串联反应构建3,4'-吡喃-螺环氧化吲哚类化合物[J]. 有机化学, 2025, 45(4): 1369-1378.
Mingmei Zhang, Feng Sha, Xin-Yan Wu. Michael Addition/Hemiketalization Cascade Reaction Between β-Dicarbonyl Compounds and Isatin-Derived α,β-Unsaturated Ketones to Construct 3,4'-Pyran-spirooxindoles[J]. Chinese Journal of Organic Chemistry, 2025, 45(4): 1369-1378.
| Entry | Catalyst | Solvent | Time/h | Yield b/% | Dr c |
|---|---|---|---|---|---|
| 1 | — | CH2Cl2 | — | N.R. | — |
| 2 | DABCO | CH2Cl2 | 42 | 80 | >20∶1 |
| 3 | Et3N | CH2Cl2 | 72 | 92 | >20∶1 |
| 4 | DIPEA | CH2Cl2 | 120 | 96 | >20∶1 |
| 5 | TMG | CH2Cl2 | 24 | 88 | >20∶1 |
| 6 | Na2CO3 | CH2Cl2 | 12 | 89 | >20∶1 |
| 7 | DMAP | CH2Cl2 | 24 | 98 | >20∶1 |
| 8 | DMAP | CHCl3 | 72 | 92 | >20∶1 |
| 9 | DMAP | DMF | 5 | 89 | >20∶1 |
| 10 | DMAP | EtOAc | 60 | 97 | >20∶1 |
| 11d | DMAP | Toluene | 72 | 89 | >20∶1 |
| 12d | DMAP | Et2O | 72 | 48 | >20∶1 |
| 13d | DMAP | THF | 72 | 76 | >20∶1 |
| 14d | DMAP | EtOH | 72 | 48 | >20∶1 |
| 15 | DMAP | MeCN | 10 | 99 | >20∶1 |
| 16e | DMAP | MeCN | 10 | 98 | >20∶1 |
| 17f | DMAP | MeCN | 8 | 96 | >20∶1 |
| 18g | DMAP | MeCN | 24 | 96 | >20∶1 |
| Entry | Catalyst | Solvent | Time/h | Yield b/% | Dr c |
|---|---|---|---|---|---|
| 1 | — | CH2Cl2 | — | N.R. | — |
| 2 | DABCO | CH2Cl2 | 42 | 80 | >20∶1 |
| 3 | Et3N | CH2Cl2 | 72 | 92 | >20∶1 |
| 4 | DIPEA | CH2Cl2 | 120 | 96 | >20∶1 |
| 5 | TMG | CH2Cl2 | 24 | 88 | >20∶1 |
| 6 | Na2CO3 | CH2Cl2 | 12 | 89 | >20∶1 |
| 7 | DMAP | CH2Cl2 | 24 | 98 | >20∶1 |
| 8 | DMAP | CHCl3 | 72 | 92 | >20∶1 |
| 9 | DMAP | DMF | 5 | 89 | >20∶1 |
| 10 | DMAP | EtOAc | 60 | 97 | >20∶1 |
| 11d | DMAP | Toluene | 72 | 89 | >20∶1 |
| 12d | DMAP | Et2O | 72 | 48 | >20∶1 |
| 13d | DMAP | THF | 72 | 76 | >20∶1 |
| 14d | DMAP | EtOH | 72 | 48 | >20∶1 |
| 15 | DMAP | MeCN | 10 | 99 | >20∶1 |
| 16e | DMAP | MeCN | 10 | 98 | >20∶1 |
| 17f | DMAP | MeCN | 8 | 96 | >20∶1 |
| 18g | DMAP | MeCN | 24 | 96 | >20∶1 |
| [1] |
For recently reviews, see: (a) Zhou, L.-M.; Qu, R.-Y.; Yang, G.-F. Expert Opin. Drug Discov. 2020, 15, 603.
pmid: 33381970 |
|
(b) Shankaraiah, N.; Bora, D.; Kaushal, A. Eur. J. Med.Chem. 2021, 215, 113263.
pmid: 33381970 |
|
|
(c) Hügel, H. M.; de Silva, N. H.; Siddiqui, A.; Blanch, E.; Lingham, A. Bioorg. Med. Chem. 2021, 43, 116270.
pmid: 33381970 |
|
|
(d) Hiesinger, K.; Dar’in, D.; Proschak, E.; Krasavin, M. J. Med. Chem. 2021, 64, 150.
doi: 10.1021/acs.jmedchem.0c01473 pmid: 33381970 |
|
|
(e) Panda, S. S.; Girgis, A. S.; Aziz, M. N.; Bekheit, M. S. Molecules 2023, 28, 618.
pmid: 33381970 |
|
|
(f) Chahat; Bhatia, R.; Kumar, B. Eur. J. Med. Chem. 2023, 247, 115020.
pmid: 33381970 |
|
|
(g) Helal, M. H.; Owda, M. E.; Mogharbel, A. T.; Hamzah Alessa, A.; Omer, N.; Abdelaziz, M. A.; Ibrahim, I.; Eliwa, E. M. Bioorg. Chem. 2024, 143, 107091.
pmid: 33381970 |
|
| [2] |
For recently reviews, see: (a) Zhu, Y.-S.; Yuan, B.-B.; Guo, J.-M.; Jin, S.-J.; Dong, H.-H.; Wang, Q.-L.; Bu, Z.-W. J. Org. Chem. 2017, 82, 5669.
pmid: 35792116 |
|
(b) Cao, Z.-Y.; Zhou, F.; Zhou, J. Acc. Chem. Res. 2018, 51, 1443.
pmid: 35792116 |
|
|
(c) Lin, Y.; Du, D.-M. Chin. J. Org. Chem. 2020, 40, 3214 (in Chinese).
pmid: 35792116 |
|
|
(林晔, 杜大明, 有机化学, 2020, 40, 3214.)
doi: 10.6023/cjoc202005065 pmid: 35792116 |
|
|
(d) Gui, H.-Z.; Wei, Y.; Shi, M. Chem.-Asian J. 2020, 15, 1225.
pmid: 35792116 |
|
|
(e) Chaudhary, A. Curr. Org. Chem. 2020, 24, 1643.
doi: 10.2174/1385272824999200622113153 pmid: 35792116 |
|
|
(f) Saranya, P. V.; Neetha, M.; Aneea, T.; Anilkumar, G. RSC Adv. 2021, 11, 7146.
doi: 10.1039/d1ra00139f pmid: 35792116 |
|
|
(g) Wang, Y.; Cobo, A. A.; Franz, A. K. Org. Chem. Front. 2021, 8, 4315.
pmid: 35792116 |
|
|
(h) Boddy, A. J.; Bull, J. A. Org. Chem. Front. 2021, 8, 1026.
pmid: 35792116 |
|
|
(i) Ganesh, M.; Suraj, S. Org. Biomol. Chem. 2022, 20, 5651.
doi: 10.1039/d2ob00767c pmid: 35792116 |
|
|
(j) Borah, B.; Veeranagaiah, N. S.; Sharma, S.; Patat, M.; Prasad, M. S.; Pallepogu, R.; Chowhan, L. R. RSC Adv. 2023, 13, 7063.
pmid: 35792116 |
|
|
(k) Borah, B.; Sharma, S.; Chowhan, L. R. Asian J. Org. Chem. 2023, 12, e202300020.
pmid: 35792116 |
|
|
(l) Wang, Q.; Liu, H.-F.; Ren, S.-Y.; He, M.-X.; Pan, Y.-M. Synthesis 2023, 55, 2873.
pmid: 35792116 |
|
|
(m) Liandi, A. R.; Cahyana, A. H.; Alfariza, D. N.; Nuraini, R.; Sari, R. W.; Wendari, T. P. Green Synth. Catal. 2024, 5, 1.
pmid: 35792116 |
|
| [3] |
(a) Ma, S.-S.; Mei, W.-L.; Guo, Z.-K.; Liu, S.-B.; Zhao, Y.-X.; Yang, D.-L.; Zeng, Y.-B.; Jiang, B.; Dai, H.-F. Org. Lett. 2013, 15, 1492.
|
|
(b) Narendraprasad, R. B.; Ramana, C. V. Tetrahedron 2017, 73, 888.
|
|
| [4] |
(a) Eastwood, P.; Gonzalez, J.; Gomez, E.; Vidal, B.; Caturla, F.; Roca, R.; Balagué, C.; Orellana, A.; Dominguez, M. Bioorg. Med. Chem. Lett. 2011, 21, 4130.
doi: 10.1016/j.bmcl.2011.05.114 pmid: 21958544 |
|
(b) Eastwood, P.; Gonzalez, J.; Gomez, E.; Caturla, F.; Balagué, C.; Orellana, A.; Dominguez, M. Bioorg. Med. Chem. Lett. 2011, 21, 5270.
doi: 10.1016/j.bmcl.2011.07.033 pmid: 21958544 |
|
|
(c) Eastwood, P.; Gonzalez, J.; Gomez, E.; Caturla, F.; Aguilar, N.; Mir, M.; Aiguadé, J.; Matassa, V.; Balagué, C.; Orellana, A.; Do- minguez, M. Bioorg. Med. Chem. Lett. 2011, 21, 6253.
doi: 10.1016/j.bmcl.2011.09.006 pmid: 21958544 |
|
| [5] |
For reviews, see: (a) Tian, J.-J.; Guo, H.-Y. Chin. J. Org. Chem. 2011, 31, 2009 (in Chinese).
|
|
(田金金, 郭红云, 有机化学, 2011, 31, 2009.)
|
|
|
(b) Cheng, D.; Ishihara, Y.; Tan, B.; Barbas III, C. F. ACS Catal. 2014, 4, 743.
|
|
| [6] |
(a) Yadav, A.; Baneree, J.; Arupula, S. K.; Mobin, S. M.; Samanta, S. Asian J. Org. Chem. 2018, 7, 1595.
|
|
(b) He, X.-L.; Zhao, H.-R.; Duan, C.-Q.; Du, W.; Chen, Y.-C. Org. Lett. 2018, 20, 804.
|
|
|
(c) Shen, Y.-B.; Li, S.-S.; Liu, X.; Yu, L.; Liu, Q.; Xiao, J. Adv. Synth. Catal. 2019, 361, 1453.
|
|
|
(d) Lin, Y.; Hou, X.-Q.; Li, B.-Y.; Du, D.-M. Adv. Synth. Catal. 2020, 362, 5728.
|
|
|
(e) Li, N.-K.; Sun, B.-B.; Chen, J.-B.; Yang, H.-D.; Wang, B.-L.; Yu, J.-Q.; Wang, X.-W.; Wang, Z. Org. Chem. Front. 2021, 8, 2009.
|
|
|
(f) Ma, Z.-W.; Chen, X.-P.; Wang, C.-C.; Wang, J.-L.; Tao, J.-C.; Lü, Q.-J. Chin. J. Org. Chem. 2022, 42, 1520 (in Chinese).
|
|
|
(马志伟, 陈晓培, 王川川, 王建玲, 陶京朝, 吕全建, 有机化学, 2022, 42, 1520.)
doi: 10.6023/cjoc202111030 |
|
|
(g) Chen, Y.-Z.; Chen, J.-J.; Zhong, L.; Zhang, Y.-L.; Zhan, R.-T.; Huang, H.-C.; Xue, Y.-B. Org. Biomol. Chem. 2024, 22, 3198.
|
|
| [7] |
Vishwanath, M.; Vinayagam, P.; Gaulapalli, V. P. R.; Kesavan, V. Asian J. Org. Chem. 2016, 5, 613.
|
| [8] |
Yin, S.-J.; Zhang, S.-Y.; Zhang, J.-Q.; Sun, B.-B.; Fan, W.-T.; Wu, B.; Wang, X.-W. RSC Adv. 2016, 6, 84248.
|
| [9] |
Li, N.; Lu, W.-J.; Gu, W.-Z.; Li, K.-L.; Li, J.-D.; Lu, Y.-M.; Zha, Z.-G.; Wang, Z.-Y. Chem. Commun. 2022, 58, 10957.
|
| [10] |
(a) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
pmid: 26200936 |
|
(b) Zhu, W.; Wang, J.; Wang, S.; Gu, Z.; Aceña, J. L.; Izawa, K.; Liu, H.; Soloshonok, V. A. J. Fluorine Chem. 2014, 167, 37.
pmid: 26200936 |
|
|
(c) Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315.
doi: 10.1021/acs.jmedchem.5b00258 pmid: 26200936 |
|
| [11] |
Sumran, G.; Jain, N.; Kumar, P.; Aggarwal, R. Chem.-Eur. J. 2024, 30, e202303599.
|
| [12] |
(a) Ren, Q.; Gao, Y.-J.; Wang, J. Org. Biomol. Chem. 2011, 9, 5297.
|
|
(b) Wang, X.-Q.; Yao, W.-J.; Yao, Z.-H.; Ma, C. J. Org. Chem. 2012, 77, 2959.
|
|
|
(c) Li, J.; Shi, W.; Yang, W.-X.; Kang, Z.-P.; Zhang, M.; Song, L.-P. RSC Adv. 2014, 4, 29549.
|
|
| [13] |
Hu, F.-L.; Wei, Y.; Shi, M. Chem. Commun. 2014, 50, 8912.
|
| [1] | 崔效源, 靳芳, 黄鑫, 秦涛, 计从斌. 铑催化硫代重氮氧化吲哚的插入反应构建苯并噻吩衍生物[J]. 有机化学, 2025, 45(7): 2501-2508. |
| [2] | 李旻昊, 王泽溟, 黄庆, 左伟伟. 钴(II)催化的酮不对称转移氢化反应[J]. 有机化学, 2025, 45(7): 2451-2460. |
| [3] | 王超, 陈洪平, 米明众, 李澳文, 齐燕, 刘永军. 锰催化的有机合成偶联反应进展[J]. 有机化学, 2025, 45(7): 2326-2334. |
| [4] | 毛婷, 曾鳞媛, 温吉林, 贾佳. 可见光介导下铱催化脂肪族α-溴代三氟甲基的脱溴环化反应[J]. 有机化学, 2025, 45(7): 2520-2528. |
| [5] | 史茜, 李忠玉, 李晗. 杂环金属铱配合物光敏剂的研究进展[J]. 有机化学, 2025, 45(7): 2389-2405. |
| [6] | 谭永波, 舒洪波, 黄华文. 光诱导N-芳基丙烯酰胺参与的吲哚酮合成研究进展[J]. 有机化学, 2025, 45(6): 2086-2108. |
| [7] | 陈刚, 陈东, 聂广杰, 李林轩, 姚辉, 王能中, 黄年玉. 有机膦催化下橙酮衍生的氮杂二烯与氨基巴豆酸酯的[2+3]环加成反应[J]. 有机化学, 2025, 45(6): 2139-2148. |
| [8] | 魏泽齐, 孙鑫浩, 黎思瑞, 宋汪泽. 基于二氢吡喃骨架修饰构建其官能化衍生物的研究进展[J]. 有机化学, 2025, 45(6): 1920-1945. |
| [9] | 钱纯节, 何博洋, 刘兴宇, 王平, 高广春, 刘石惠. 铜/Selectfluor共催化的苄基碳氢键Ritter胺基化反应[J]. 有机化学, 2025, 45(6): 2181-2188. |
| [10] | 王霜, 毛羊杰, 娄绍杰, 许丹倩. 基于氧化型导向基团的不对称C—H键官能团化反应研究进展[J]. 有机化学, 2025, 45(6): 1961-1994. |
| [11] | 姚嫣, 付年凯. 光电化学金属催化研究进展[J]. 有机化学, 2025, 45(6): 1819-1837. |
| [12] | 周强, 杨宝臻, 郝贵林, 罗木鹏, 曹石, 赵蓓, 袁华, 王守国. 铑(III)催化的非活化烯烃与α-重氮羰基化合物的对映选择性烯丙位C—H键烷基化反应[J]. 有机化学, 2025, 45(6): 2109-2120. |
| [13] | 梁珍, 徐维焱, 陈毅, 邱化玉, 赵也哲, 沈佳斌, 王民. 铱催化N-芳基-2-氨基吡啶和碳酸亚乙烯酯合成吲哚衍生物[J]. 有机化学, 2025, 45(6): 2149-2156. |
| [14] | 任天磊, 汪鑫, 丛欢. 蒽光二聚体衍生的手性单膦配体: 借助化学拆分的制备路线和不对称烯丙基胺化的合成应用[J]. 有机化学, 2025, 45(6): 2208-2221. |
| [15] | 张丛玉, 陈晓琦, 孟凡涛, 王海营, 郝文娟, 姜波. 金自接力催化1,3-烯炔醋酸酯与环醚缩醛的环化/亲核取代反应研究[J]. 有机化学, 2025, 45(6): 2199-2207. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||