有机化学 ›› 2025, Vol. 45 ›› Issue (3): 837-851.DOI: 10.6023/cjoc202410006 上一篇 下一篇
综述与进展
收稿日期:2024-10-12
修回日期:2024-12-01
发布日期:2024-12-19
基金资助:
Yunshan Lia,b, Yuxin Zhanga, Yefeng Tanga( )
)
Received:2024-10-12
Revised:2024-12-01
Published:2024-12-19
Contact:
* E-mail: Supported by:文章分享

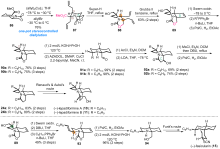
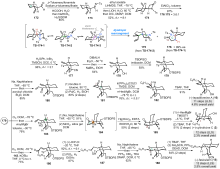
Cylindricines, lepadiformines和fasicularin是一系列具有氢化吡咯[2,1-j]喹啉(6/6/5)或氢化吡啶[2,1-j]喹啉(6/6/6)核心骨架的三环海洋生物碱. 该类天然产物具有新颖的化学结构和广泛的生物学活性, 近40年来吸引了众多合成化学家的研究兴趣. 综述了自2017年以来关于该类天然产物的合成研究进展, 对每个合成中的关键构环反应进行了分析和总结.
李芸杉, 张钰欣, 唐叶峰. 三环海洋生物碱Cylindricines/Lepadiformines/Fasicularin的合成进展[J]. 有机化学, 2025, 45(3): 837-851.
Yunshan Li, Yuxin Zhang, Yefeng Tang. Recent Advance in the Synthesis of Tricyclic Marine Alkaloids: Cylindricines, Lepadiformines, and Fasicularin[J]. Chinese Journal of Organic Chemistry, 2025, 45(3): 837-851.


| [1] |
(a) Blackman, A. J.; Li, C.; Hockless, D. C. R.; Skelton, B. W.; White, A. H. Tetrahedron 1993, 49, 8645.
|
|
(b) Biard, J. F.; Guyot, S.; Roussakis, C.; Verbist, J. F.; Vercauteren, J.; Weber, J. F.; Boukef, K. Tetrahedron Lett. 1994, 35, 2691.
|
|
|
(c) Li, C. P.; Blackman, A. J. Aust. J. Chem. 1994, 47, 1355.
|
|
|
(d) Li, C. P.; Blackman, A. J. Aust. J. Chem. 1995, 48, 955.
|
|
|
(e) Patil, A. D.; Freyer, A. J.; Reichwein, R.; Carte, B.; Killmer, L. B.; Faucette, L.; Johnson, R. K.; Faulkner, D. J. Tetrahedron Lett. 1997, 38, 363.
|
|
| [2] |
(a) Jugé, M.; Grimaud, N.; Biard, J.-F.; Sauviat, M.-P.; Nabil, M.; Verbist, J.-F.; Petit, J.-Y. Toxicon 2001, 39, 1231.
|
|
(b) Sauviat, M. P.; Vercauteren, J.; Grimaud, N.; Juge, M.; Nabil, M.; Petit, J. Y.; Biard, J. F. J. Nat. Prod. 2006, 69, 558.
|
|
| [3] |
Dutta, S.; Abe, H.; Aoyagi, S.; Kibayashi, C.; Gates, K. S. J. Am. Chem. Soc. 2005, 127, 15004.
|
| [4] |
Snider, B. B.; Liu, T. J. Org. Chem. 1997, 62, 5630.
|
| [5] |
Weinreb, S. M. Chem. Rev. 2006, 106, 2531.
|
| [6] |
Chiba, S.; Kaga, A. Synthesis 2017, 50, 685.
|
| [7] |
Wu, J.-L.; Chiou, W.-H. J. Org. Chem. 2020, 85, 9051.
|
| [8] |
Wang, Y.-T.; Wu, J.-L.; Chiou, W.-H. Org. Lett. 2022, 24, 5957.
|
| [9] |
Pastor, A.; Prinsloo, R.; Burford, K. N.; Macdonald, A. R.; Parvez, M.; Gendy, C.; Back, T. G. J. Org. Chem. 2023, 88, 13813.
|
| [10] |
Hiraoka, S.; Matsumoto, T.; Matsuzaka, K.; Sato, T.; Chida, N. Angew. Chem., Int. Ed. 2019, 58, 4381.
|
| [11] |
Yamamoto, S.; Komiya, Y.; Kobayashi, A.; Minamikawa, R.; Oishi, T.; Sato, T.; Chida, N. Org. Lett. 2019, 21, 1868.
|
| [12] |
Lin, H.-C.; Xie, P.-P.; Dai, Z.-Y.; Zhang, S.-Q.; Wang, P.-S.; Chen, Y.-G.; Wang, T.-C.; Hong, X.; Gong, L.-Z. J. Am. Chem. Soc. 2019, 141, 5824.
|
| [13] |
Takashima, K.; Hayakawa, D.; Gouda, H.; Toyooka, N. J. Org. Chem. 2019, 84, 5222.
|
| [14] |
Shimomura, M.; Sato, M.; Azuma, H.; Sakata, J.; Tokuyama, H. Org. Lett. 2020, 22, 3313.
|
| [15] |
Huang, Y.-H.; Liu, Z.-J.; Huang, P.-Q. Org. Chem. Front. 2022, 9, 58.
|
| [16] |
Piccichè, M.; Pinto, A.; Griera, R.; Bosch, J.; Amat, M. Org. Lett. 2022, 24, 5356.
|
| [17] |
(a) Yoshimura, A.; Hanzawa, R.; Fuwa, H. Org. Lett. 2022, 24, 6237.
|
|
(b) Hanzawa, R.; Fuwa, H. Org. Lett. 2023, 25, 1984.
|
|
|
(c) Takatori, Y.; Fuwa, H. J. Org. Chem. 2024, 89, 11693.
|
|
| [18] |
Dukes, D. M.; Atanassov, V. K.; Smith, J. M. Chem. Sci. 2024, 15, 16554.
|
| [19] |
Li, Y.; Zhang, J.; Chen, Y.; Pang, J.; Chen, Y.; Tang, Y. Angew. Chem., Int. Ed. 2024, e202414985.
|
| [1] | 李闯, 张成, 刘小宇, 秦勇. 二萜生物碱全合成研究进展[J]. 有机化学, 2025, 45(3): 881-895. |
| [2] | 杨庆星, 刘璇, 马硕, 李欣欣, 马东旭, 徐涛. 多卤代海洋来源天然产物全合成[J]. 有机化学, 2025, 45(3): 764-803. |
| [3] | 杨云博, 丁寒锋. 四奎烷及类四奎烷全合成研究进展[J]. 有机化学, 2025, 45(3): 725-747. |
| [4] | 文国恩, 谷硕, 何海兵, 高栓虎. Kuroshine类生物碱的合成研究[J]. 有机化学, 2025, 45(3): 977-987. |
| [5] | 斯绪格, 蔡泉. 抗血管生成天然产物Penduliflaworosin的不对称全合成及结构修正[J]. 有机化学, 2025, 45(3): 959-968. |
| [6] | 杨帆, 濮留洋, 谢建华, 周其林. 四环高原阿朴啡碱(+)-Crociflorinone及(+)-6a-epi-Crociflorinone的不对称全合成[J]. 有机化学, 2025, 45(3): 969-976. |
| [7] | 谢应, 付绍敏, 刘波. 酰基自由基化学在天然产物全合成中的应用[J]. 有机化学, 2025, 45(3): 852-861. |
| [8] | 高志宇, 路雪娜, 李奕晴, 任丽, 郝宏东. 灵芝杂萜的全合成研究进展[J]. 有机化学, 2025, 45(3): 814-836. |
| [9] | 易九州, 火亮, 陈金燕, 刘勐, 李辉林, 厍学功. 保护基效应对几类天然产物全合成的影响[J]. 有机化学, 2025, 45(3): 1030-1039. |
| [10] | 孟龙, 乔金宝, 赵玉明. 尼亚那属二萜天然产物的全合成研究进展[J]. 有机化学, 2025, 45(3): 804-813. |
| [11] | 张晓锋, Aggeliki Roumana, 毛海康, 徐晶. 虎皮楠生物碱Daphniglaucin C的AB环系合成[J]. 有机化学, 2025, 45(3): 925-932. |
| [12] | 龙涛, 何述钟, 李超. 自由基-极性交叉转化反应在天然产物全合成中的研究进展[J]. 有机化学, 2025, 45(3): 748-763. |
| [13] | 韩守乐, 娄明亮, 刘晓磊, 李根, 王馨, 吴青翠, 齐湘兵. 吲哚发散性氢化及其在(±)-α-和γ-Lycoranes的全合成中的应用[J]. 有机化学, 2025, 45(3): 913-924. |
| [14] | 陈杰, 李俊, 龙先文, 申海香, 邓军. Wagner-Meerwein重排反应在天然产物全合成中的应用[J]. 有机化学, 2025, 45(3): 896-912. |
| [15] | 毛海康, 徐晶. 虎皮楠生物碱全合成研究进展[J]. 有机化学, 2025, 45(3): 866-880. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||