有机化学 ›› 2025, Vol. 45 ›› Issue (8): 2746-2766.DOI: 10.6023/cjoc202412020 上一篇 下一篇
综述与进展
收稿日期:2024-12-24
修回日期:2025-03-05
发布日期:2025-03-25
基金资助:
Baichuan Mo*( ), Tingting Li, Fang Wang, Xiaojie Li, Darifu Ba*(
), Tingting Li, Fang Wang, Xiaojie Li, Darifu Ba*( )
)
Received:2024-12-24
Revised:2025-03-05
Published:2025-03-25
Contact:
*E-mail:MoBaiChuan2023@163.com;18800465508@163.com
Supported by:文章分享
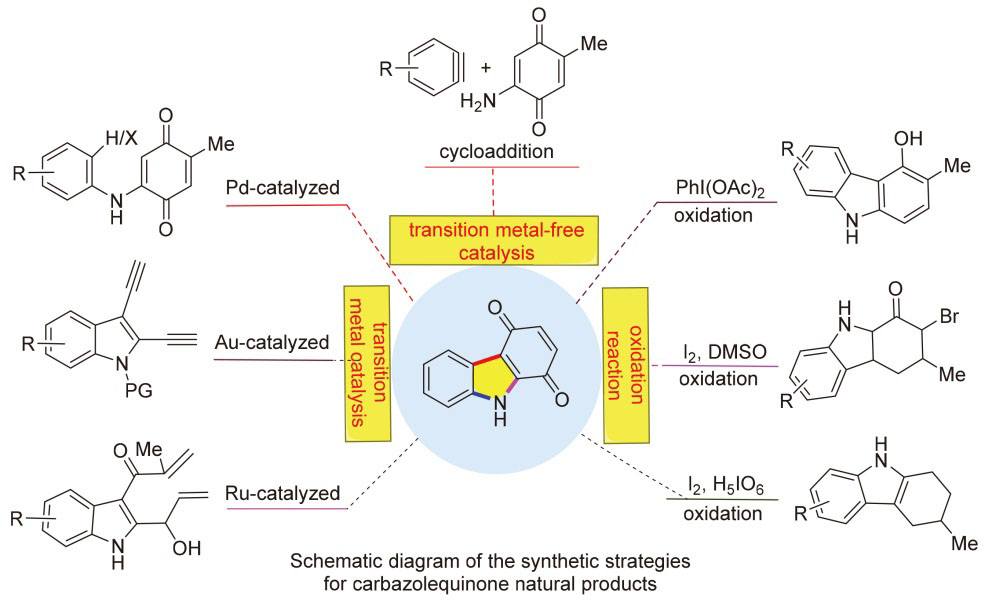
咔唑醌天然产物因其良好的抗疟疾、抗肿瘤和抗病毒等生物活性受到了广泛关注, 成为药物化学领域的重要研究对象. 综述了近三十年来咔唑醌类化合物的合成策略, 主要包括过渡金属催化(钯、铜、钌等)、无过渡金属催化及氧化反应等方法, 这些策略实现了咔唑醌类化合物的高效简洁合成. 同时展望了未来的研究方向, 包括开发更高效的催化剂体系、拓展反应底物范围及发展绿色合成方法, 以进一步提升咔唑醌类化合物在药物研发领域的应用潜力. 最后深入探索其构效关系, 为基于咔唑醌母核的新药创制提供新的策略与思路.
莫百川, 李婷婷, 王芳, 李晓杰, 巴达日夫. 咔唑醌天然产物的合成研究进展[J]. 有机化学, 2025, 45(8): 2746-2766.
Baichuan Mo, Tingting Li, Fang Wang, Xiaojie Li, Darifu Ba. Recent Progress in the Synthesis of Carbazolequinone Natural Products[J]. Chinese Journal of Organic Chemistry, 2025, 45(8): 2746-2766.




| [1] |
|
| [2] |
doi: 10.1021/cr200447s pmid: 22480243 |
| [3] |
pmid: 2599008 |
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
pmid: 15369400 |
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
doi: 10.1021/jo302821v pmid: 23421392 |
| [18] |
|
| [19] |
doi: 10.1039/c4ob00493k pmid: 24881674 |
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
doi: 10.1021/acs.joc.7b02101 pmid: 29086565 |
| [24] |
|
| [25] |
|
|
(赵子萌, 贺耘, 中国科技论文在线精品论文, 2018, 11, 1086.)
|
|
| [26] |
|
|
(张坤, 徐红进, 刘咨博, 宋传君, 有机化学, 2019, 39, 1142.)
doi: 10.6023/cjoc201812005 |
|
| [27] |
|
|
(李雪, 宋子锐, 陈鑫, 蔡轶超, 刘雅婕, 陈春霞, 彭进松, 有机化学, 2020, 40, 950.)
doi: 10.6023/cjoc201909040 |
|
| [28] |
doi: S0960-894X(19)30418-4 pmid: 31253531 |
| [29] |
|
| [30] |
|
| [31] |
|
|
(韩阳, 姜为超, 张靖, 彭进松, 陈春霞, 有机化学, 2022, 42, 266.)
doi: 10.6023/cjoc202104037 |
|
| [32] |
|
| [33] |
|
| [34] |
doi: 10.1039/c5ob01766a pmid: 26395099 |
| [35] |
|
| [36] |
|
| [37] |
doi: S0223-5234(16)30473-1 pmid: 27318980 |
| [38] |
doi: S0223-5234(17)30345-8 pmid: 28477443 |
| [39] |
doi: 10.1021/ol4015738 pmid: 23819770 |
| [40] |
|
| [41] |
doi: 10.1021/jo502509s pmid: 25525818 |
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
doi: 10.1021/jo402593w pmid: 24372379 |
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [1] | 王烁圻, 王成明. 一种毛兰素的高效合成方法[J]. 有机化学, 2025, 45(7): 2625-2629. |
| [2] | 丁一鸣, 张庭荣, 张泾渭, 邓军. 三尖杉二萜类天然产物的全合成研究进展[J]. 有机化学, 2025, 45(6): 2048-2073. |
| [3] | 何卫刚, 刘亚东, 邓迎开, 孙先宇. Malagasy生物碱的合成研究进展[J]. 有机化学, 2025, 45(4): 1153-1165. |
| [4] | 韩守乐, 娄明亮, 刘晓磊, 李根, 王馨, 吴青翠, 齐湘兵. 吲哚发散性氢化及其在(±)-α-和γ-Lycoranes的全合成中的应用[J]. 有机化学, 2025, 45(3): 913-924. |
| [5] | 毛海康, 徐晶. 虎皮楠生物碱全合成研究进展[J]. 有机化学, 2025, 45(3): 866-880. |
| [6] | 陈杰, 李俊, 龙先文, 申海香, 邓军. Wagner-Meerwein重排反应在天然产物全合成中的应用[J]. 有机化学, 2025, 45(3): 896-912. |
| [7] | 孟龙, 乔金宝, 赵玉明. 尼亚那属二萜天然产物的全合成研究进展[J]. 有机化学, 2025, 45(3): 804-813. |
| [8] | 张晓锋, Aggeliki Roumana, 毛海康, 徐晶. 虎皮楠生物碱Daphniglaucin C的AB环系合成[J]. 有机化学, 2025, 45(3): 925-932. |
| [9] | 文国恩, 谷硕, 何海兵, 高栓虎. Kuroshine类生物碱的合成研究[J]. 有机化学, 2025, 45(3): 977-987. |
| [10] | 斯绪格, 蔡泉. 抗血管生成天然产物Penduliflaworosin的不对称全合成及结构修正[J]. 有机化学, 2025, 45(3): 959-968. |
| [11] | 杨帆, 濮留洋, 谢建华, 周其林. 四环高原阿朴啡碱(+)-Crociflorinone及(+)-6a-epi-Crociflorinone的不对称全合成[J]. 有机化学, 2025, 45(3): 969-976. |
| [12] | 谢应, 付绍敏, 刘波. 酰基自由基化学在天然产物全合成中的应用[J]. 有机化学, 2025, 45(3): 852-861. |
| [13] | 李芸杉, 张钰欣, 唐叶峰. 三环海洋生物碱Cylindricines/Lepadiformines/Fasicularin的合成进展[J]. 有机化学, 2025, 45(3): 837-851. |
| [14] | 高志宇, 路雪娜, 李奕晴, 任丽, 郝宏东. 灵芝杂萜的全合成研究进展[J]. 有机化学, 2025, 45(3): 814-836. |
| [15] | 李闯, 张成, 刘小宇, 秦勇. 二萜生物碱全合成研究进展[J]. 有机化学, 2025, 45(3): 881-895. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||