有机化学 ›› 2025, Vol. 45 ›› Issue (9): 3213-3243.DOI: 10.6023/cjoc202503010 上一篇 下一篇
综述与进展
收稿日期:2025-03-10
修回日期:2025-04-21
发布日期:2025-05-30
基金资助:
Huanle Li, Qi Pan, Shaojie Lou, Yangjie Mao*( ), Danqian Xu*(
), Danqian Xu*( )
)
Received:2025-03-10
Revised:2025-04-21
Published:2025-05-30
Contact:
E-mail: Supported by:文章分享

烷基胺类化合物是重要的有机分子, 对其不同位点的碳氢键进行精准转化是当前有机合成化学的研究热点之一. 氢原子迁移(HAT)作为一种温和可控的碳氢键活化策略, 近些年来受到了极高的关注并被广泛用于胺类分子的选择性碳氢键转化. 通过分子间或分子内HAT过程可以精准导向攫取胺类分子中特定位点的氢原子并实现后续转化, 为复杂胺类功能分子的高效构筑提供了全新的合成思路. 总结了基于上述策略的胺类分子碳氢键选择性官能团化反应进展, 对其中的区域、化学和立体选择性调控机制以及反应机理进行评述, 分析存在的局限性以及展望未来研究方向.
李欢乐, 潘其, 娄绍杰, 毛羊杰, 许丹倩. 基于氢原子迁移(HAT)过程的胺类化合物选择性C—H键转化反应研究进展[J]. 有机化学, 2025, 45(9): 3213-3243.
Huanle Li, Qi Pan, Shaojie Lou, Yangjie Mao, Danqian Xu. Recent Advances in Selective C—H Bond Functionalization of Amines Through Hydrogen Atom Transfer (HAT) Process[J]. Chinese Journal of Organic Chemistry, 2025, 45(9): 3213-3243.

| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
(a)
|
|
(b)
|
|
| [51] |
|
| [1] |
|
| [2] |
(a)
|
|
(b)
|
|
| [3] |
|
| [4] |
|
|
韩叶强, 史炳锋, 化学学报, 2023, 81, 1522).
doi: 10.6023/A23070336 |
|
| [5] |
|
| [6] |
(a)
|
|
(b)
|
|
|
(c)
|
|
| [7] |
(a)
|
| [52] |
|
| [53] |
doi: 10.1039/d0sc04881j pmid: 34123240 |
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
doi: 10.1021/jacs.0c11103 pmid: 33249845 |
| [58] |
doi: 10.1021/jacs.2c05266 pmid: 35820104 |
| [59] |
|
| [60] |
|
| [61] |
|
|
(刘慧英, 吴中天, 李昊天, 吴新鑫, 有机化学, 2025, 45, 297.)
doi: 10.6023/cjoc202406034 |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
(a)
|
|
(b)
|
|
| [66] |
|
| [67] |
|
| [7] |
(王淼, 黄雅豪, 胡鹏, 有机化学, 2025, 45, 477.)
doi: 10.6023/cjoc202407027 |
|
(b)
|
|
|
(c)
|
|
| [8] |
(a)
pmid: 25345694 |
|
(b)
pmid: 25345694 |
|
|
(c)
doi: 10.1002/chem.201403951 pmid: 25345694 |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
|
(杨俊峰, 赵艳秋, 时磊, 有机化学, 2025, 45, 559.)
doi: 10.6023/cjoc202406050 |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
(a)
pmid: 39364063 |
| [13] |
doi: 10.1016/j.chempr.2022.07.022 pmid: 36571075 |
| [14] |
|
| [15] |
(a)
|
|
(b)
|
|
|
(c)
|
|
| [16] |
|
| [17] |
|
| [75] |
(b)
doi: 10.1038/s44160-024-00536-2 pmid: 39364063 |
|
(c)
doi: 10.1038/s44160-024-00507-7 pmid: 39364063 |
|
|
(d)
pmid: 39364063 |
|
| [76] |
(a)
|
|
(b)
|
|
| [18] |
|
| [19] |
(a)
pmid: 33739109 |
|
(b)
doi: 10.1021/acs.chemrev.0c00736 pmid: 33739109 |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
(a)
|
|
(b)
|
|
| [24] |
(a)
|
|
(b)
|
|
| [25] |
|
| [26] |
|
| [77] |
|
| [78] |
doi: 10.1038/s41467-019-12722-4 pmid: 31628325 |
| [79] |
doi: 10.1021/acs.orglett.0c02008 pmid: 32697587 |
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
doi: 10.1021/jacs.9b07014 pmid: 31478370 |
| [27] |
|
|
(王晓琴, 许盛, 平媛媛, 孔望清, 有机化学, 2025, 45, 383.)
|
|
| [28] |
|
| [29] |
|
| [30] |
doi: 10.1126/science.abh2623 pmid: 33888639 |
| [31] |
|
| [32] |
|
| [33] |
(a)
|
|
(b)
|
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
(a)
doi: 10.1021/jacs.4c02284 pmid: 38568080 |
|
(b)
pmid: 38568080 |
|
| [42] |
doi: 10.1021/jacs.2c08989 pmid: 36375080 |
| [43] |
|
| [44] |
|
| [45] |
doi: 10.1021/jacs.9b05850 pmid: 31280562 |
| [46] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
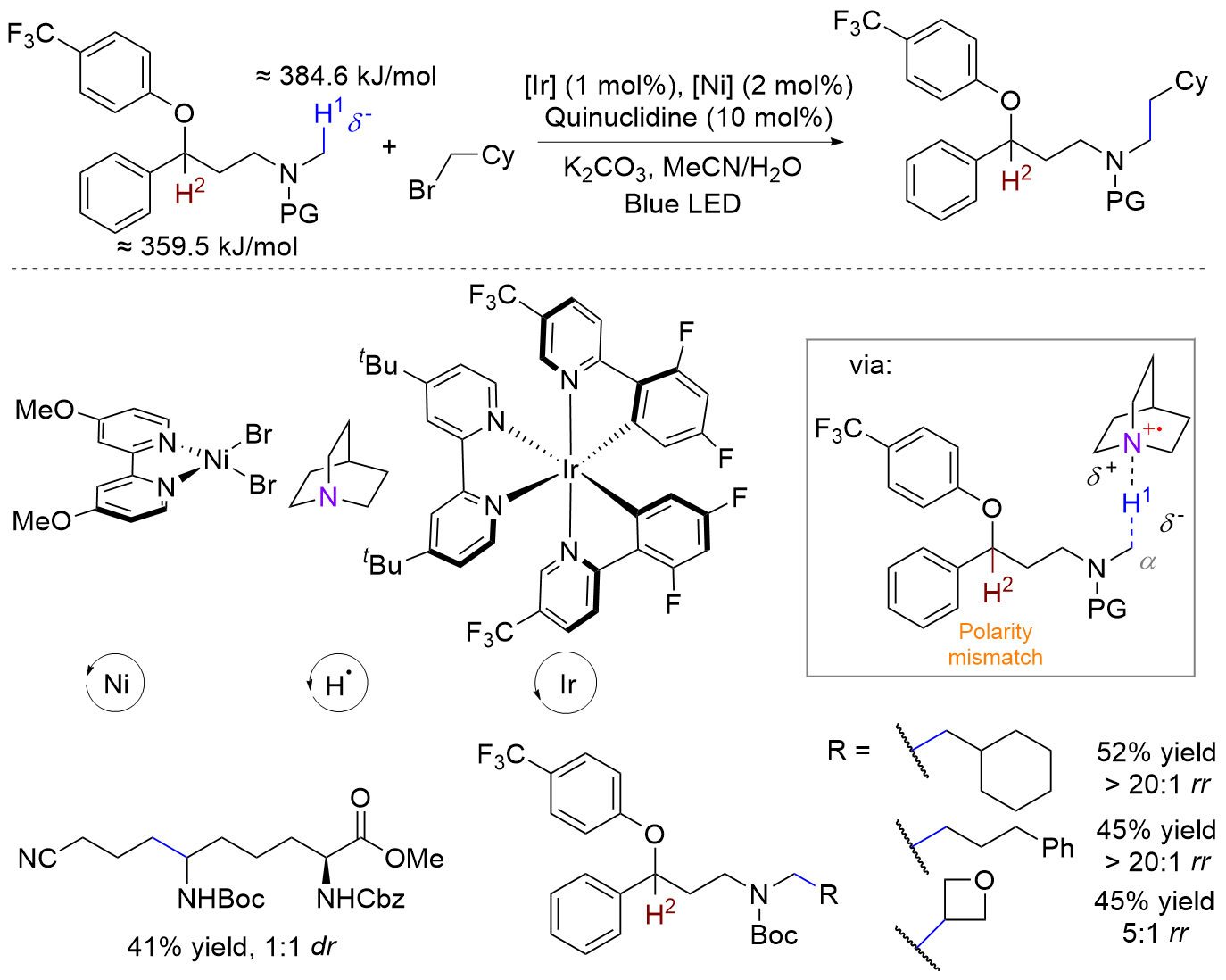
(a)
pmid: 39361751 |
|
(b)
doi: 10.1126/science.adq8029 pmid: 39361751 |
|
|
(c)
pmid: 39361751 |
| [1] | 杨威, 王兵兵, 徐小惠, 孙钟, 唐翠曼, 刘佳琦. 仿生材料聚多巴胺在有机转化中的应用进展[J]. 有机化学, 2025, 45(9): 3314-3325. |
| [2] | 贾晨旭, 安聪好, 黄军. 铜催化C—O偶联制备间苯氧基苯甲醛[J]. 有机化学, 2025, 45(9): 3412-3419. |
| [3] | 任博文, 方通昌, 刘超. 可见光诱导双硼化合成烷基偕二硼酸酯★[J]. 有机化学, 2025, 45(9): 3343-3350. |
| [4] | 汤敏, 张斌, 王秋实, 方超华, 胡立威, 关丽萍. 基于包含吲哚环、苯并噻吩环的单胺氧化酶和胆碱酯酶抑制活性的查尔酮衍生物设计、合成及生物活性研究[J]. 有机化学, 2025, 45(8): 2989-3003. |
| [5] | 刘丽强, 南军, 段兴宇, 王昊龙, 蔡巷, 王梓诚, 张恩泽, 孙彦民. 三齿P配体钌络合物催化脂肪醇胺N-烷基化反应研究[J]. 有机化学, 2025, 45(8): 2983-2988. |
| [6] | 解人杰, 谢复开, 孙然, 王欣, 王钰佳, 李蕾, 王贺. 电子供体-受体复合物(EDA)介导N-芳基丙烯酰胺与芳基硫鎓盐的自由基环化反应[J]. 有机化学, 2025, 45(8): 2913-2922. |
| [7] | 王小燕, 徐长明. Brønsted酸催化烯丙醇的伯胺化反应[J]. 有机化学, 2025, 45(8): 2932-2937. |
| [8] | 缪尹盛, 张磊, 陈建圻, 王丽丽, 段征. 双季鏻盐催化芳基炔烃与N-亚胺(异)喹啉叶立德合成吡唑并(异)喹啉衍生物[J]. 有机化学, 2025, 45(8): 2876-2884. |
| [9] | 沈健, 王彦博, 吴正兴, 徐德锋, 张万斌. α,β-不饱和γ-内酯(酰胺)的不对称催化合成[J]. 有机化学, 2025, 45(8): 2637-2659. |
| [10] | 张斌斌, 刘露娜, 竺杨彬, 邹东. 自由基介导的硝基芳烃活化反应研究进展[J]. 有机化学, 2025, 45(8): 2698-2725. |
| [11] | 林依鸣, 岳佳怡, 张春燕, 陈艺林, 朱红平. 芳基铜(I)与烷基硅胺的反应: 无限链芳基铜(I)、多核硅胺铜(I)及纳米粒子铜(0)的合成和表征[J]. 有机化学, 2025, 45(8): 2848-2853. |
| [12] | 陶星慧, 郭凯悦, 张继坦, 谢美华, 吴佳萍. 钯催化溴代芳烃邻位碳氢胺化/碳氢芳基化关环反应合成官能化氮杂䓬酮和菲啶[J]. 有机化学, 2025, 45(8): 2825-2835. |
| [13] | 陈晓培, 王清龙, 侯学会, 王川川, 马志伟, 张京玉. CF3-亚胺酰基亚砜叶立德在环化反应中的应用研究进展[J]. 有机化学, 2025, 45(8): 2796-2814. |
| [14] | 韩哲, 崔银, 丁成荣, 杨建, 张国富. “SO2F”源直接构建磺酰氟官能团的合成应用研究进展[J]. 有机化学, 2025, 45(7): 2245-2264. |
| [15] | 何华西, 周和烨, 刘彬. 苝酰亚胺大环的研究进展[J]. 有机化学, 2025, 45(7): 2265-2282. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||