有机化学 ›› 2026, Vol. 46 ›› Issue (1): 1-20.DOI: 10.6023/cjoc202506007 上一篇 下一篇
综述与进展
姜志洁a,†, 陈锦秀b,c,†, 朱晓菲d, 邓鑫浩c, 严琼姣c,*( ), 汪伟b,*(
), 汪伟b,*( ), 周慧b,*(
), 周慧b,*( )
)
收稿日期:2025-07-15
修回日期:2025-08-19
发布日期:2025-09-11
作者简介:† 共同第一作者
基金资助:
Zhijie Jianga, Jinxiu Chenb,c, Xiaofei Zhud, Xinhao Dengc, Qiongjiao Yanc,*( ), Wei Wangb,*(
), Wei Wangb,*( ), Hui Zhoub,*(
), Hui Zhoub,*( )
)
Received:2025-07-15
Revised:2025-08-19
Published:2025-09-11
Contact:
* E-mail: 875531154@qq.com;
wang520520wei@163.com;
huizhou@ccnu.edu.cn
About author:† The authors contributed equally to this work
Supported by:文章分享
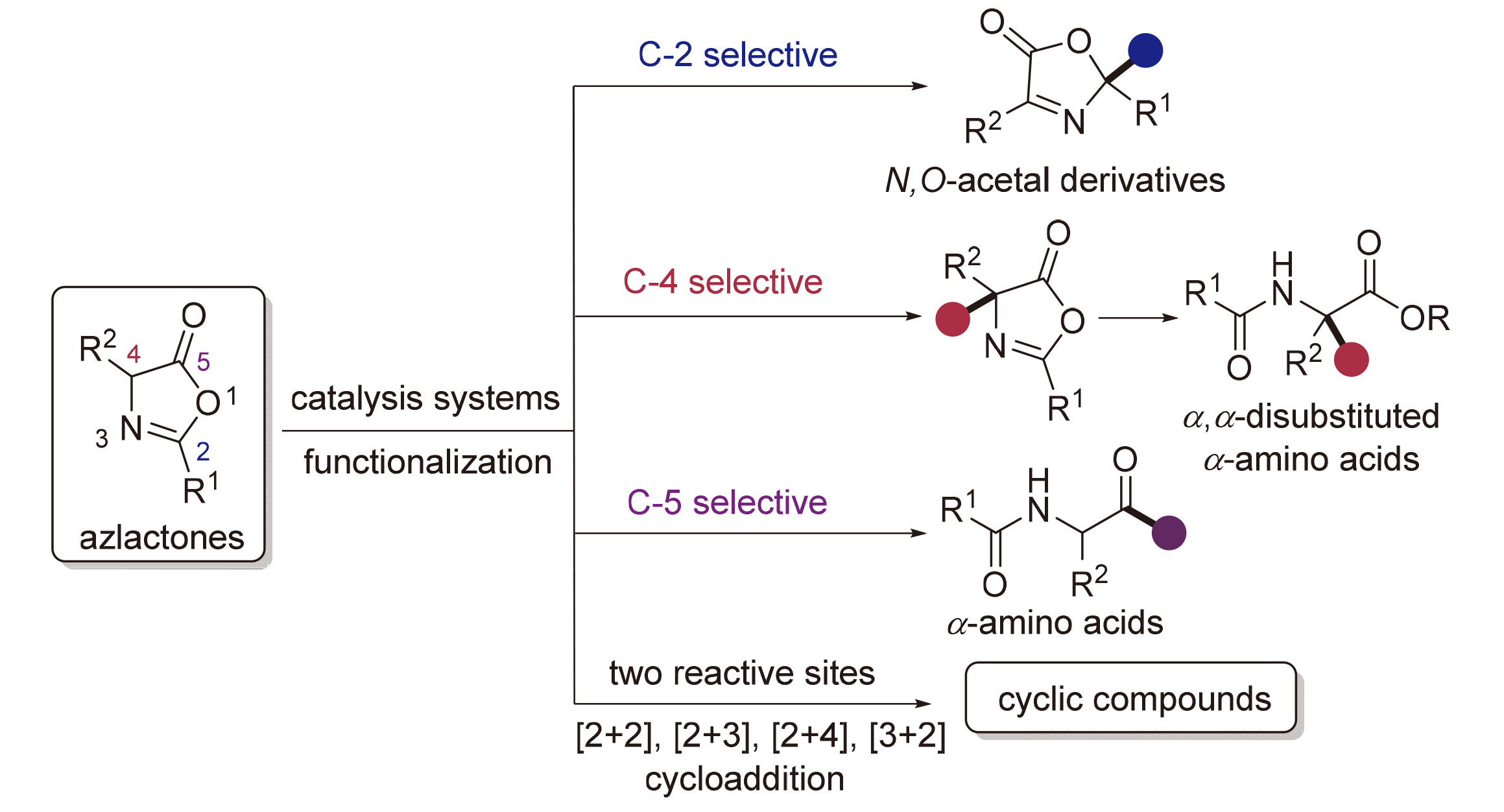
噁唑酮是合成α,α-二取代氨基酸、N,O-缩醛类化合物以及杂环化合物的重要前体, 在有机合成中的应用越来越广泛. 近年来基于噁唑酮的官能团转化合成了一系列重要有机化合物, 特别是利用其不对称转化得到了重要手性化合物. 因此, 涉及噁唑酮转化的反应引起了越来越多科研工作者关注. 基于噁唑酮多活性位点的特性, 按其不同活性位点分类综述了噁唑酮参与的各种有机反应, 包括C-2位、C-4位和C-5位官能团化以及多反应位点的环加成反应. 同时也重点介绍了本课题组最近开发的基于邻苯二甲酰羟胺活性酯原位形成噁唑酮合成手性非天然氨基酸的方法, 与已有的噁唑酮官能团化反应形成优势互补, 促进该领域的蓬勃发展. 最后对该领域所面临的挑战和机遇进行了展望和探讨.
姜志洁, 陈锦秀, 朱晓菲, 邓鑫浩, 严琼姣, 汪伟, 周慧. 噁唑酮不同活性位点的官能团化反应研究进展[J]. 有机化学, 2026, 46(1): 1-20.
Zhijie Jiang, Jinxiu Chen, Xiaofei Zhu, Xinhao Deng, Qiongjiao Yan, Wei Wang, Hui Zhou. Recent Advances on the Functionalization of Azlactones at Different Reactive Sites[J]. Chinese Journal of Organic Chemistry, 2026, 46(1): 1-20.
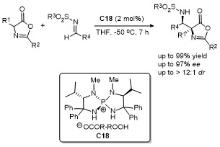
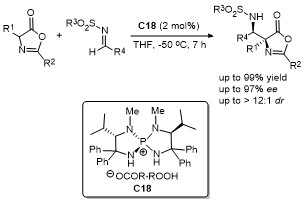
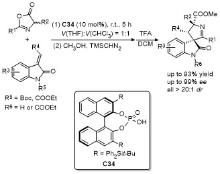
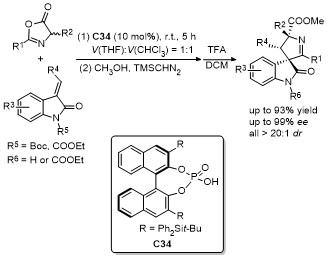
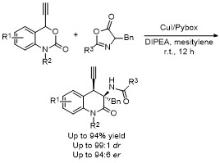
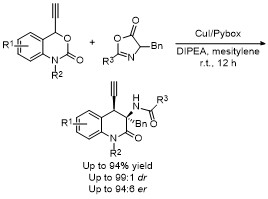
| [33] |
doi: 10.1002/anie.v48:47 |
| [34] |
doi: 10.1021/ja208156z |
| [35] |
doi: 10.1002/anie.v58.9 |
| [36] |
doi: 10.1021/ja310209g pmid: 23145913 |
| [37] |
doi: 10.1021/ja510603w pmid: 25423341 |
| [38] |
|
| [39] |
doi: 10.1002/adsc.v358.1 |
| [1] |
doi: 10.3390/molecules27030671 |
| [2] |
doi: 10.1039/b511113g |
| [3] |
doi: 10.1126/science.1176758 pmid: 19713492 |
| [4] |
doi: 10.1021/ja9097803 pmid: 20131827 |
| [5] |
doi: 10.1039/C5OB00121H |
| [6] |
doi: 10.1021/jacs.5b10524 |
| [7] |
doi: 10.1002/anie.v56.29 |
| [8] |
doi: 10.1039/C9CC01450K |
| [40] |
doi: 10.1039/C7CC01715D |
| [41] |
doi: 10.1021/acs.orglett.8b03020 |
| [42] |
doi: 10.1021/acs.orglett.1c01193 |
| [43] |
doi: 10.1021/ja806311e pmid: 18834197 |
| [44] |
doi: 10.1002/anie.v51.17 |
| [45] |
doi: 10.1021/acs.joc.7b02556 |
| [46] |
doi: 10.1021/acs.joc.9b03000 |
| [47] |
doi: 10.1021/ol702941f |
| [9] |
doi: 10.1039/D0QO00348D |
| [10] |
doi: 10.1021/acs.orglett.1c00259 |
| [11] |
doi: 10.1039/D1QO00569C |
| [12] |
|
| [13] |
|
| [14] |
doi: 10.1021/ja992754n |
| [15] |
doi: 10.1021/ja020290e |
| [16] |
doi: 10.1021/ja029190z |
| [17] |
doi: 10.1021/jo2002962 |
| [48] |
doi: 10.1002/anie.v50.52 |
| [49] |
pmid: 26918108 |
| [50] |
doi: 10.1039/D2CC01656G |
| [51] |
doi: 10.1039/d0ob00476f pmid: 32236278 |
| [52] |
doi: 10.1021/jo9803380 |
| [53] |
doi: 10.1002/anie.v44:5 |
| [54] |
|
| [55] |
doi: 10.1021/jo402828f |
| [18] |
doi: 10.1021/ja311363a |
| [19] |
doi: 10.1039/D0CC00265H |
| [20] |
doi: 10.1021/jacs.8b13582 |
| [21] |
doi: 10.1039/D2CC00328G |
| [22] |
doi: 10.1021/acs.joc.3c01152 |
| [23] |
|
| [24] |
doi: 10.1021/ja301461p |
| [25] |
|
| [56] |
doi: 10.1002/ajoc.v5.7 |
| [57] |
pmid: 17900190 |
| [58] |
doi: 10.1021/ja1046928 |
| [59] |
doi: 10.1021/ja404379n |
| [60] |
doi: 10.1002/anie.v52.33 |
| [61] |
doi: 10.1039/C5CC08989A |
| [62] |
doi: 10.1021/acs.joc.6b00078 |
| [26] |
doi: 10.1038/s41467-023-41956-6 |
| [27] |
doi: 10.1021/ol500053c pmid: 24568135 |
| [28] |
doi: 10.1039/D1OB00582K |
| [29] |
doi: 10.1021/ol034570q |
| [30] |
doi: 10.1002/adsc.v356.11/12 |
| [31] |
doi: 10.1016/S0040-4039(00)89379-X |
| [32] |
doi: 10.1021/ja982890c |
| [63] |
doi: 10.1002/anie.v57.19 |
| [64] |
doi: 10.1002/anie.v57.18 |
| [65] |
doi: 10.1016/j.tetlet.2019.06.041 |
| [66] |
doi: 10.1039/D0CC06418A |
| [67] |
doi: 10.1039/D1QO00948F |
| [68] |
doi: 10.1039/D0OB02388D |
| [69] |
doi: 10.1039/D2OB00618A |
| [70] |
doi: 10.1021/acscatal.3c03814 |
| [71] |
doi: 10.1039/D4QO02257B |
| [1] | 任钶, 张光露, 牛轶凡, 王晓萌, 陈灿玉, 蒋敏. 光催化合成含氮杂芳环非天然氨基酸[J]. 有机化学, 2026, 46(1): 215-224. |
| [2] | 王宇, 黄鸿坤, 凌春, 陈恬, 姚华, 严兆华. 苯乙烯化合物与咪唑并吡啶化合物和硫氰化钾的四组分羟基/巯基化反应[J]. 有机化学, 2026, 46(1): 181-188. |
| [3] | 彭澳霈, 李扬, 朱园园, 吴广文, 古双喜. 席夫碱型荧光探针对氨基酸化学选择性识别研究进展[J]. 有机化学, 2025, 45(9): 3301-3313. |
| [4] | 李欢乐, 潘其, 娄绍杰, 毛羊杰, 许丹倩. 基于氢原子迁移(HAT)过程的胺类化合物选择性C—H键转化反应研究进展[J]. 有机化学, 2025, 45(9): 3213-3243. |
| [5] | 谢昊池, 秦永康, 杨婷, 李湖进, 孙佳嘉, 钱明成, 赵帅, 侯亚男, 陈新. 可见光介导的烯醇和重氮化合物之间的O—H键官能团化反应[J]. 有机化学, 2025, 45(8): 3004-3016. |
| [6] | 贺重隆, 周有康, 段新华, 刘乐. 官能团迁移策略在光驱动不饱和烃双官能团化中的应用[J]. 有机化学, 2025, 45(5): 1478-1508. |
| [7] | 袁晨晖, 焦雷. 手性配体在钯催化配位辅助对映选择性C(sp3)—H键官能团化反应中的应用[J]. 有机化学, 2025, 45(2): 602-619. |
| [8] | 沈佳斌, 沈超, 章鹏飞. 可见光介导的羰基α位C—H官能团化反应合成萘咪酮类衍生物[J]. 有机化学, 2025, 45(2): 677-685. |
| [9] | 王淼, 黄雅豪, 胡鹏. 氢原子转移介导的烷烃C(sp3)—H选择性官能团化研究进展[J]. 有机化学, 2025, 45(2): 477-497. |
| [10] | 冯天惠, 任志强, 高甜莉, 韩波, 郭蕊丽, 马豪杰, 王记江, 张玉琦. 光促进铜催化异噁唑酮与醇反应合成异肟酸酯[J]. 有机化学, 2025, 45(12): 4346-4353. |
| [11] | 孙良龙, 孙凯. 1,3-烯炔双官能团化反应研究进展[J]. 有机化学, 2025, 45(10): 3655-3671. |
| [12] | 张晨晨, 丁清杰, 吕琪妍, 马春华, 姜玉钦, 於兵. 基于配体-金属电荷转移的铁催化光致脱羧官能团化反应[J]. 有机化学, 2025, 45(10): 3517-3533. |
| [13] | 焦燕燕, 王飞格, 肖建, 安孝德. 醇的脱羟官能团化研究进展[J]. 有机化学, 2025, 45(10): 3587-3612. |
| [14] | 张腾飞, 常喆, 陈春霞, 彭进松. 过渡金属催化氮原子α位Csp3—H键官能团化反应研究进展[J]. 有机化学, 2025, 45(1): 168-188. |
| [15] | 王君伟, 薛皓, 曲英瑜, 姜若楠, 闫法超, 刘会. 过渡金属催化联烯胺类化合物的碳氢化反应研究进展[J]. 有机化学, 2025, 45(1): 151-167. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||