有机化学 ›› 2025, Vol. 45 ›› Issue (2): 677-685.DOI: 10.6023/cjoc202406051 上一篇 下一篇
研究论文
收稿日期:2024-06-30
修回日期:2024-08-31
发布日期:2024-09-26
基金资助:
Jiabin Shena,b,c, Chao Shenb, Pengfei Zhangc( )
)
Received:2024-06-30
Revised:2024-08-31
Published:2024-09-26
Contact:
*E-mail: pfzhang@hznu.edu.cn
Supported by:文章分享
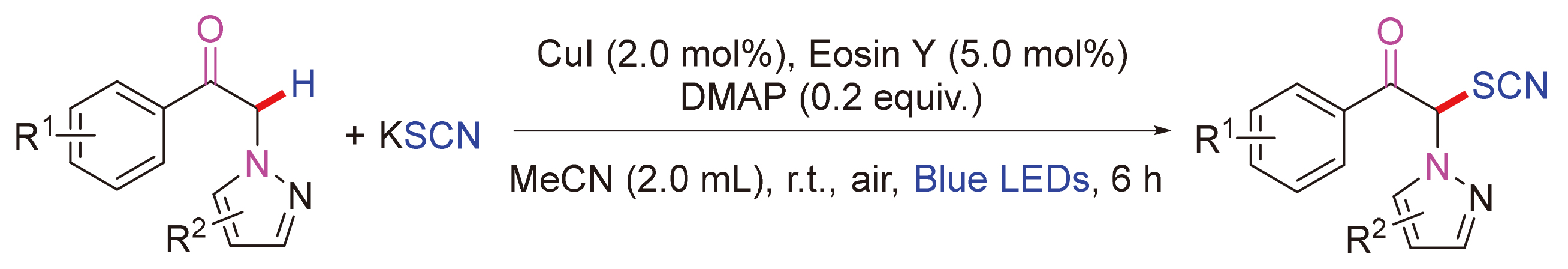
开发了一种以曙红Y (Esoin Y)为光催化剂的可见光诱导的普适和实用的羰基α位C—H官能团化策略. 这种温和的转化为制备具有潜在生物活性的萘咪酮类衍生物提供了一种操作简单、底物范围广且高原子经济的高效便捷的方法. 机制研究表明该策略涉及自由基反应机制.
沈佳斌, 沈超, 章鹏飞. 可见光介导的羰基α位C—H官能团化反应合成萘咪酮类衍生物[J]. 有机化学, 2025, 45(2): 677-685.
Jiabin Shen, Chao Shen, Pengfei Zhang. Synthesis of Nafimidone Derivatives by Visible-Light-Induced α-C—H Functionalization of Carbonyl[J]. Chinese Journal of Organic Chemistry, 2025, 45(2): 677-685.
| Entry | Metal-catalyst | Photocatalyst | Base | Solvent | Yieldb/% |
|---|---|---|---|---|---|
| 1 | CuI | Fluorescein | Et3N | MeCN | 65 |
| 2 | CuCl | Fluorescein | Et3N | MeCN | 54 |
| 3 | CuCl2 | Fluorescein | Et3N | MeCN | 43 |
| 4 | Cu2O | Fluorescein | Et3N | MeCN | 14 |
| 5 | CuO | Fluorescein | Et3N | MeCN | 17 |
| 6 | — | Fluorescein | Et3N | MeCN | Trace |
| 7 | CuI | Rose Bengal | Et3N | MeCN | 54 |
| 8 | CuI | 9-Fluorenone | Et3N | MeCN | 71 |
| 9 | CuI | Mes-Acr+ | Et3N | MeCN | 43 |
| 10 | CuI | — | Et3N | MeCN | Trace |
| 11 | CuI | Eosin Y | Et3N | MeCN | 76 |
| 12 | CuI | Eosin Y | DMAP | MeCN | 87 |
| 13 | CuI | Eosin Y | NaOH | MeCN | 64 |
| 14 | CuI | Eosin Y | KOH | MeCN | 67 |
| 15 | CuI | Eosin Y | K2HPO4 | MeCN | 51 |
| 16 | CuI | Eosin Y | NaHCO3 | MeCN | 47 |
| 17 | CuI | Eosin Y | DMAP | THF | 52 |
| 18 | CuI | Eosin Y | DMAP | DCE | 63 |
| 19 | CuI | Eosin Y | DMAP | DMSO | 74 |
| 20 | CuI | Eosin Y | DMAP | DMF | 68 |
| 21 | CuI | Eosin Y | DMAP | Dioxane | 56 |
| 22c | CuI | Eosin Y | DMAP | MeCN | 86 |
| 23d | CuI | Eosin Y | DMAP | MeCN | 52 |
| 24e | CuI | Eosin Y | DMAP | MeCN | 87 |
| 25f | CuI | Eosin Y | DMAP | MeCN | 64 |
| 26g | CuI | Eosin Y | DMAP | MeCN | 87 |
| 27h | CuI | Eosin Y | DMAP | MeCN | 61 |
| 28i | CuI | Eosin Y | DMAP | MeCN | Trace |
| 29j | CuI | Eosin Y | DMAP | MeCN | Trace |
| Entry | Metal-catalyst | Photocatalyst | Base | Solvent | Yieldb/% |
|---|---|---|---|---|---|
| 1 | CuI | Fluorescein | Et3N | MeCN | 65 |
| 2 | CuCl | Fluorescein | Et3N | MeCN | 54 |
| 3 | CuCl2 | Fluorescein | Et3N | MeCN | 43 |
| 4 | Cu2O | Fluorescein | Et3N | MeCN | 14 |
| 5 | CuO | Fluorescein | Et3N | MeCN | 17 |
| 6 | — | Fluorescein | Et3N | MeCN | Trace |
| 7 | CuI | Rose Bengal | Et3N | MeCN | 54 |
| 8 | CuI | 9-Fluorenone | Et3N | MeCN | 71 |
| 9 | CuI | Mes-Acr+ | Et3N | MeCN | 43 |
| 10 | CuI | — | Et3N | MeCN | Trace |
| 11 | CuI | Eosin Y | Et3N | MeCN | 76 |
| 12 | CuI | Eosin Y | DMAP | MeCN | 87 |
| 13 | CuI | Eosin Y | NaOH | MeCN | 64 |
| 14 | CuI | Eosin Y | KOH | MeCN | 67 |
| 15 | CuI | Eosin Y | K2HPO4 | MeCN | 51 |
| 16 | CuI | Eosin Y | NaHCO3 | MeCN | 47 |
| 17 | CuI | Eosin Y | DMAP | THF | 52 |
| 18 | CuI | Eosin Y | DMAP | DCE | 63 |
| 19 | CuI | Eosin Y | DMAP | DMSO | 74 |
| 20 | CuI | Eosin Y | DMAP | DMF | 68 |
| 21 | CuI | Eosin Y | DMAP | Dioxane | 56 |
| 22c | CuI | Eosin Y | DMAP | MeCN | 86 |
| 23d | CuI | Eosin Y | DMAP | MeCN | 52 |
| 24e | CuI | Eosin Y | DMAP | MeCN | 87 |
| 25f | CuI | Eosin Y | DMAP | MeCN | 64 |
| 26g | CuI | Eosin Y | DMAP | MeCN | 87 |
| 27h | CuI | Eosin Y | DMAP | MeCN | 61 |
| 28i | CuI | Eosin Y | DMAP | MeCN | Trace |
| 29j | CuI | Eosin Y | DMAP | MeCN | Trace |
| [1] |
Acar, M. F.; Sari, S.; Dalkara, S. Drug Dev. Res. 2019, 80, 606.
|
| [2] |
Özdemir, Z.; Sari, S.; Karakurt, A.; Dalkara, S. Drug Dev. Res. 2019, 80, 269.
|
| [3] |
Sari, S.; Karakurt, A.; Uslu, H.; Kaynak, F. B.; Çalıs, Ü.; Dalkara, S. Eur. J. Med. Chem. 2016, 124, 407.
|
| [4] |
Yamada, K.; Yajima, O.; Yoshizawa, Y.; Oh, K. Bioorg. Med. Chem. 2013, 21, 2451.
|
| [5] |
Weitz, J. I.; Chan, N. C. Arterioscler., Thromb., Vasc. Biol. 2019, 39, 7.
|
| [6] |
Lu, X.; He, S.; Li, Q.; Yang, H.; Jiang, X.; Lin, H.; Chen, Y.; Qu, W.; Feng, F.; Bian, Y.; Zhou, Y.; Sun, H. Bioorg. Med. Chem. 2018, 26, 1665.
|
| [7] |
Macarini, A. F.; Sobrinho, T. U. C.; Rizzi, G. W.; Corrêa, R. Med. Chem. Res. 2019, 28, 1235.
doi: 10.1007/s00044-019-02368-8 |
| [8] |
Rilatt, I.; Mirabel, E.; Le Grand, B.; Perez, M. Bioorg. Med. Chem. 2010, 20, 903.
|
| [9] |
(a) Ouyang, W.-T.; Jiang, J.; Jiang, Y.-F.; Li, T.; Liu, Y.-Y.; Ji, H.-T.; Ou, L.-J.; He, W.-M. Chin. Chem. Lett. 2024, 35, 110038.
|
|
(b) Hou, J.-C.; Ji, H.-T.; Lu, Y.-H.; Wang, J.-S.; Xu, Y.-D.; Zeng, Y.-Y.; He, W.-M. Chin. Chem. Lett. 2024, 35, 109514.
|
|
|
(c) Chen, X.; Ouyang, W.-T.; Li, X.; He, W.-M. Chin. J. Org. Chem. 2023, 43, 4213 (in Chinese).
|
|
|
(陈祥, 欧阳文韬, 李潇, 何卫民, 有机化学, 2023, 43, 4213.)
doi: 10.6023/cjoc202307026 |
|
|
(d) Guo, Y.; Zhu, J.; Wang, Y.; Li, Y.; Hu, H.; Zhang, P.; Xu, J.; Li, W. ACS Catal. 2024, 14, 619.
|
|
|
(e) Zhang, L.; Wang, Y.; Shen, J.; Xu, H.; Shen, C. Org. Chem. Front. 2024, 11, 2727.
|
|
|
(f) Liu, Y.; Pang, Q.; Chen, B.; Zhu, Z.; Shen, J.; Zhang, P. Mol. Catal. 2024, 569, 114592.
|
|
|
(g) Yi, R.-N.; He, W.-M. Chin. Chem. Lett. 2024, 35, 110194.
|
|
| [10] |
(a) Li, L.; Pang, Q.; Chen, B.; Liu, Y.; Zhao, Y.; Wu, J.; Ge, K.; Shen, J.; Zhang, P. Org. Lett. 2024, 26, 7060.
|
|
(b) Xu, J.; Zhang, Y.; Xu, R.; Wang, Y.; Shen, J.; Li, W. Org. Chem. Front. 2024, 11, 5122.
|
|
|
(c) Shen, J.; Yang, Y.; Chen, C.; Xu, H.; Shen, C.; Zhang, P. Org. Chem. Front. 2024, 11, 1758.
|
|
|
(d) Yi, R.; He, W. Chin. J. Org. Chem. 2024, 44, 1035 (in Chinese).
|
|
|
(易荣楠, 何卫民, 有机化学, 2024, 44, 1035.)
doi: 10.6023/cjoc202400014 |
|
|
(e) Ma, C.; Luo, H.; Zhang, F.; Guo, D.; Chen, S.; Wang, F. Chin. J. Org. Chem. 2024, 44, 216 (in Chinese).
|
|
|
(马翠云, 罗海澜, 张福华, 郭丹, 陈树兴, 王飞, 有机化学, 2024, 44, 216.)
doi: 10.6023/cjoc202304028 |
|
|
(f) Xu, J.; Hong, Y.; Xu, R.; Wang, Y.; Guo, Y.; Shen, J.; Zhao, Y.; Li, W. Org. Chem. Front. 2024, 11, 6166.
|
|
| [11] |
Tian, H.; Xu, W.; Liu, Y.; Wang, Q. Org. Lett. 2020, 22, 5005.
doi: 10.1021/acs.orglett.0c01574 pmid: 32610920 |
| [12] |
Wang, J.; Ye, Y.; Sang, T.; Zhou, C.; Bao, X.; Yuan, Y.; Huo, C. Org. Lett. 2022, 24, 7577.
|
| [13] |
Wang, Y.-L.; Zhang, T.-X.; Zhang, X.-M.; Sun, H.-Y.; Leng, J.-Y.; Li, Y.-M. Chin. J. Org. Chem. 2023, 43, 4284 (in Chinese).
|
|
(王永玲, 张铁欣, 张栩铭, 孙晗扬, 冷津瑶, 李亚明, 有机化学, 2023, 43, 4284.)
doi: 10.6023/cjoc202304020 |
|
| [14] |
Shen, J.; Wang, Z.; Zhang, Y.; Xu, J.; Shen, C.; Zhang, P. Org. Chem. Front. 2023, 10, 605.
|
| [15] |
(a) Wang, Z.; Hu, L.; Zhao, M.; Dai, L.; Hrynsphan, D.; Tatsiana, S.; Chen, J. BioChar 2022, 4, 28.
|
|
(b) Lv, S.; Zheng, F.; Wang, Z.; Hayat, K.; Veiga, M. C.; Kennes, C.; Chen, J. Sci. Total Environ. 2024, 908, 168339.
|
|
|
(c) Guo, T.; Yue, H.; Ma, C.; Li, S.; Chen, J.; Li, W.; Zhao, J. Biorem. J. 2022, 26, 171.
|
|
|
(d) Yao, J.; Mei, Y.; Yuan, B.; Zheng, F.; Wang, Z.; Chen, J. Environ. Res. 2024, 241, 117613.
|
|
|
(e) Wang, Z.; Chen, C.; Liu, H.; Hrynshpan, D.; Savitskaya, T.; Chen, J.; Chen, J. Ecotoxicol. Environ. Saf. 2019, 183, 109507.
|
|
|
(f) Wang, Z.; Fu, W.; Hu, L.; Zhao, M.; Guo, T.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Sci. Total Environ. 2021, 781, 146686.
|
|
|
(g) Wang, Z.; Chen, C.; Liu, H.; Hrynshpan, D.; Savitskaya, T.; Chen, J.; Chen, J. Sci. Total Environ. 2020, 708, 135063.
|
|
|
(h) Wang, Z.; Dai, L.; Yao, J.; Guo, T.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Chemosphere 2021, 281, 130718.
|
|
| [16] |
Yuan, P.-F.; Zhang, Q.-B.; Jin, X.-L.; Lei, W.-L.; Wu, L.-Z.; Liu, Q. Green Chem. 2018, 20, 5464.
|
| [17] |
(a) Shen, J.; Wang, Z.; Zhang, Y.; Xu, J.; Liu, X.; Shen, C.; Zhang, P. Org. Lett. 2022, 24, 3614.
|
|
(b) Shen, J.; Wang, Z.; Shen, C.; Zhang, P. Mol. Catal. 2023, 537, 112950.
|
|
|
(c) Wang, Z.; Chen, B.; Pang, Q.; Xu, J.; Shen, J.; Xu, J.; Li, W. Mol. Catal. 2024, 559, 114060.
|
|
|
(d) Wang, Z.; Li, J.; Liu, Y.; Chen, Q.; Zhang, P.; Wu, J. Mol. Catal. 2023, 540, 113038.
|
|
| [18] |
(a) Shen, J.; Xu, J.; He, L.; Liang, C.; Li, W. Chin. Chem. Lett. 2022, 33, 1227.
|
|
(b) Shen, J.; Xu, J.; He, L.; Ouyang, Y.; Huang, L.; Li, W.; Zhu, Q. Zhang, P. Org. Lett. 2021, 23, 1204.
|
|
|
(c) Liang, C.; Wang, Z.; Chen, Q.; Wu, J.; Zhang, P.; Shen, J. Mol. Catal. 2024, 558, 114024.
|
|
|
(d) Li, L.; Liu, Y.; Li, J.; Chen, Q.; Zhang, P.; Shen, J.; Wu, J. Adv. Synth. Catal. 2023, 365, 3112.
|
|
|
(e) Zhu, J.; Hong, Y.; Wang, Y.; Guo, Y.; Zhang, Y.; Ni, Z.; Li, W.; Xu, J. ACS Catal. 2024, 14, 6247.
|
| [1] | 段琛, 沈思语, 赵钰琦, 刘跃, 李薪宇, 张礼智, 李文静. 可见光驱动下蒽醌催化苄基C—H键在水中的氧化反应[J]. 有机化学, 2025, 45(4): 1352-1359. |
| [2] | 赵佳, 甘秋云, 袁耀锋. 自由基磺酰氟化反应研究进展[J]. 有机化学, 2025, 45(4): 1206-1222. |
| [3] | 傅艳华, 徐畅, 张超, 王怡莎, 冯高峰. 可见光诱导铁催化氮杂环的羟甲基化[J]. 有机化学, 2024, 44(7): 2265-2273. |
| [4] | 曹香雪, 贾雅会, 殷世纪, 徐亮, 韦玉, 宋欢欢. 可见光诱导二氢喹唑啉酮碳碳键断裂与三氟甲基取代烯烃的脱氟烷基化反应研究[J]. 有机化学, 2024, 44(5): 1549-1557. |
| [5] | 徐辉, 蒋慧娴, 阚磊, 徐佩, 朱旭. 可见光诱导甲酸盐参与的炔烃氢羧基化反应[J]. 有机化学, 2024, 44(10): 3241-3248. |
| [6] | 张澳龙, 杨晗, 程佩栋, 姚阳, 孙松. 可见光促进烯烃与丙二酸酯、CO2的碳-羧化反应研究[J]. 有机化学, 2024, 44(10): 3159-3168. |
| [7] | 朱彦硕, 王红言, 舒朋华, 张克娜, 王琪琳. 烷氧自由基引发1,5-氢原子转移实现C(sp3)—H键官能团化的研究进展[J]. 有机化学, 2024, 44(1): 1-17. |
| [8] | 童红恩, 郭宏宇, 周荣. 可见光促进惰性碳-氢键对羰基的加成反应进展[J]. 有机化学, 2024, 44(1): 54-69. |
| [9] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [10] | 杨晓娜, 郭宏宇, 周荣. 可见光促进有机硅化合物参与的化学转化[J]. 有机化学, 2023, 43(8): 2720-2742. |
| [11] | 许晓萍, 张翼飞, 莫小渝, 江俊. 铑催化3-重氮吲哚-2-亚胺与吡唑啉酮的C—H官能团化反应制备3-吡唑基吲哚[J]. 有机化学, 2023, 43(7): 2519-2527. |
| [12] | 高艳华, 张银潘, 张妍, 宋涛, 杨勇. 可见光驱动表面富含氧空位Nb2O5催化醇氧化反应[J]. 有机化学, 2023, 43(7): 2572-2579. |
| [13] | 赵金晓, 魏彤辉, 柯森, 李毅. 可见光催化合成二氟烷基取代的多环吲哚化合物[J]. 有机化学, 2023, 43(3): 1102-1114. |
| [14] | 赵瑜, 段玉荣, 史时辉, 白育斌, 黄亮珠, 杨晓军, 张琰图, 冯彬, 张建波, 张秋禹. 可见光促进高价碘(III)试剂参与反应的研究进展[J]. 有机化学, 2023, 43(12): 4106-4140. |
| [15] | 秦思凝. 芳香卤代物C—S偶联反应的研究进展[J]. 有机化学, 2023, 43(11): 3761-3783. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||