有机化学 ›› 2026, Vol. 46 ›› Issue (1): 225-232.DOI: 10.6023/cjoc202506013 上一篇 下一篇
研究论文
冯发秀, 徐世杰*( ), 王雪, 杨卓然, 王文君, 黄申林, 张小祥*(
), 王雪, 杨卓然, 王文君, 黄申林, 张小祥*( )
)
收稿日期:2025-06-06
修回日期:2025-07-23
发布日期:2025-09-11
基金资助:
Faxiu Feng, Shijie Xu*( ), Xue Wang, Zhuoran Yang, Wenjun Wang, Shenlin Huang, Xiaoxiang Zhang*(
), Xue Wang, Zhuoran Yang, Wenjun Wang, Shenlin Huang, Xiaoxiang Zhang*( )
)
Received:2025-06-06
Revised:2025-07-23
Published:2025-09-11
Contact:
* E-mail: 1094171748@qq.com;
zhangxiaoxiang@njfu.edu.cn
Supported by:文章分享
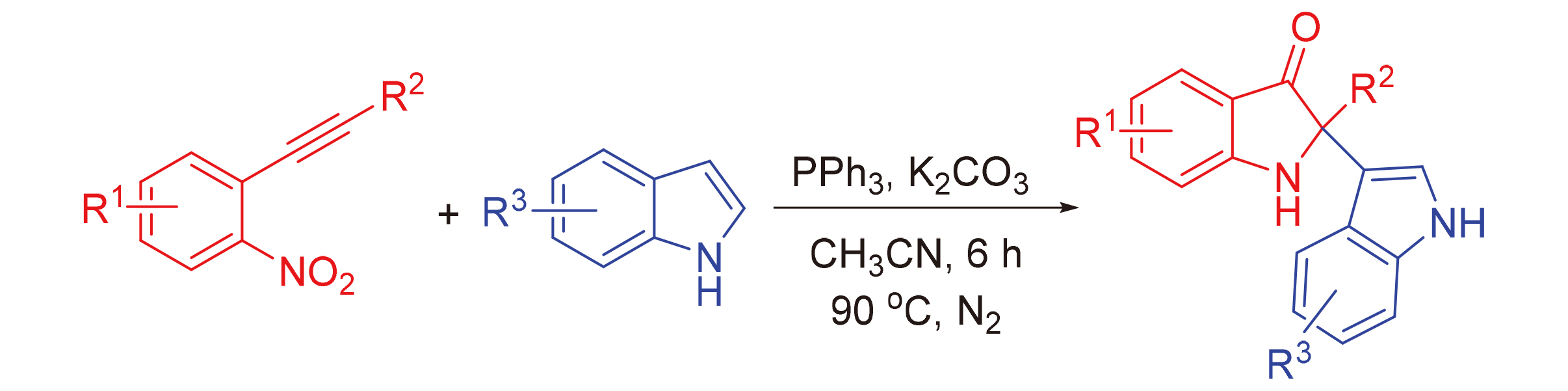
报道了一种在碱性条件下通过2-炔基硝基苯和吲哚制备吲哚啉-3-酮衍生物的有效合成方法. 在该反应中, 由PPh3引发Wittig过程, 继而在碱作用下对吲哚进行活化, 以98%的产率得到C(2)-吲哚取代的吲哚啉-3-酮. 该反应可在不含过渡金属的条件下进行, 并通过对邻炔基硝基苯进行修饰, 合成了多种官能团化的吲哚啉-3-酮衍生物.
冯发秀, 徐世杰, 王雪, 杨卓然, 王文君, 黄申林, 张小祥. PPh3介导2-炔基硝基苯与吲哚一锅法合成吲哚啉-3-酮[J]. 有机化学, 2026, 46(1): 225-232.
Faxiu Feng, Shijie Xu, Xue Wang, Zhuoran Yang, Wenjun Wang, Shenlin Huang, Xiaoxiang Zhang. PPh3-Mediated One-Pot Synthesis of Indolin-3-ones from 2-Alkynylnitrobenzenes and Indoles[J]. Chinese Journal of Organic Chemistry, 2026, 46(1): 225-232.
| Entry | Base/equiv. | Solvent | PPh3/equiv. | 2a/equiv. | Yieldb/% |
|---|---|---|---|---|---|
| 1 | K2CO3 (3) | CH3CN | 10 | 5 | 95 |
| 2 | DBU (3) | CH3CN | 10 | 5 | 27 |
| 3 | NaOH (3) | CH3CN | 10 | 5 | Trace |
| 4 | Cs2CO3 (3) | CH3CN | 10 | 5 | 80 |
| 5 | t-BuOK (3) | CH3CN | 10 | 5 | Trace |
| 6 | K2CO3 (5) | CH3CN | 10 | 5 | 97 |
| 7 | K2CO3 (2) | CH3CN | 10 | 5 | 89 |
| 8 | K2CO3 (5) | CH3CN | 10 | 2 | 90 |
| 9 | K2CO3 (5) | CH3CN | 10 | 10 | 97 |
| 10 | K2CO3 (5) | Et3N | 10 | 5 | Trace |
| 11 | K2CO3 (5) | H2O | 10 | 5 | 38 |
| 12 | K2CO3 (5) | DMSO | 10 | 5 | 25 |
| 13 | K2CO3 (5) | 1,4-Dioxane | 10 | 5 | 13 |
| 14c | K2CO3 (5) | CH3CN | 10 | 5 | 60 |
| 15d | K2CO3 (5) | CH3CN | 10 | 5 | Trace |
| 16 | K2CO3 (5) | CH3CN | 5 | 5 | 78 |
| 17e | K2CO3 (5) | CH3CN | 5 | 5 | 98 |
| 18 | — | CH3CN | 5 | 5 | Trace |
| 19 | K2CO3 (5) | CH3CN | 2 | 5 | 55 |
| Entry | Base/equiv. | Solvent | PPh3/equiv. | 2a/equiv. | Yieldb/% |
|---|---|---|---|---|---|
| 1 | K2CO3 (3) | CH3CN | 10 | 5 | 95 |
| 2 | DBU (3) | CH3CN | 10 | 5 | 27 |
| 3 | NaOH (3) | CH3CN | 10 | 5 | Trace |
| 4 | Cs2CO3 (3) | CH3CN | 10 | 5 | 80 |
| 5 | t-BuOK (3) | CH3CN | 10 | 5 | Trace |
| 6 | K2CO3 (5) | CH3CN | 10 | 5 | 97 |
| 7 | K2CO3 (2) | CH3CN | 10 | 5 | 89 |
| 8 | K2CO3 (5) | CH3CN | 10 | 2 | 90 |
| 9 | K2CO3 (5) | CH3CN | 10 | 10 | 97 |
| 10 | K2CO3 (5) | Et3N | 10 | 5 | Trace |
| 11 | K2CO3 (5) | H2O | 10 | 5 | 38 |
| 12 | K2CO3 (5) | DMSO | 10 | 5 | 25 |
| 13 | K2CO3 (5) | 1,4-Dioxane | 10 | 5 | 13 |
| 14c | K2CO3 (5) | CH3CN | 10 | 5 | 60 |
| 15d | K2CO3 (5) | CH3CN | 10 | 5 | Trace |
| 16 | K2CO3 (5) | CH3CN | 5 | 5 | 78 |
| 17e | K2CO3 (5) | CH3CN | 5 | 5 | 98 |
| 18 | — | CH3CN | 5 | 5 | Trace |
| 19 | K2CO3 (5) | CH3CN | 2 | 5 | 55 |
| [1] |
(a)
doi: 10.1002/ejoc.v2021.1 pmid: 35425542 |
|
(b)
doi: 10.1039/D0OB01368D pmid: 35425542 |
|
|
(c)
doi: 10.1021/acs.joc.1c01726 pmid: 35425542 |
|
|
(d)
doi: 10.1039/d2ra00400c pmid: 35425542 |
|
| [2] |
(a)
doi: 10.1039/C9CC00434C pmid: 15305207 |
|
(b)
doi: 10.1016/j.cclet.2014.11.011 pmid: 15305207 |
|
|
(c)
pmid: 15305207 |
|
|
(d)
doi: 10.1021/acs.orglett.2c00738 pmid: 15305207 |
|
|
(e)
doi: 10.1021/acs.joc.1c00931 pmid: 15305207 |
|
|
(f)
doi: 10.1021/acs.joc.9b02771 pmid: 15305207 |
|
| [3] |
(a)
doi: 10.1002/anie.v57.49 pmid: 35424637 |
|
(b)
doi: 10.1002/chem.v26.63 pmid: 35424637 |
|
|
(c)
doi: 10.1039/C6RA06361F pmid: 35424637 |
|
|
(d)
doi: 10.1039/d1ra08858k pmid: 35424637 |
|
|
(e)
pmid: 35424637 |
|
|
(f)
doi: 10.1039/D2RA00612J pmid: 35424637 |
|
| [4] |
(a)
pmid: 11878966 |
|
(b)
pmid: 11878966 |
|
|
(c)
pmid: 11878966 |
|
|
(d)
doi: 10.1039/D1QO01602D pmid: 11878966 |
|
|
(e)
doi: 10.1039/D0QO01157F pmid: 11878966 |
|
| [5] |
(a)
doi: 10.1002/adsc.v362.13 |
|
(b)
doi: 10.1021/acs.joc.2c01593 |
|
|
(c)
|
|
|
(d)
doi: 10.1039/D4OB01703J |
|
|
(e)
doi: 10.1039/D4QO01904K |
|
|
(f)
doi: 10.1002/anie.v54.38 |
|
|
(g)
|
|
|
(郑灏宁, 刘金宇, 化学学报, 2024, 82, 641.)
doi: 10.6023/A24030094 |
|
|
(h)
|
|
|
(高炜洋, 邓伟超, 高扬, 梁仁校, 贾义霞, 化学学报, 2024, 82, 1.)
doi: 10.6023/A23100472 |
|
|
(i)
|
|
|
(黄佳鑫, 刘敏, 徐辉, 戴辉雄, 化学学报, 2024, 82, 565.)
doi: 10.6023/A24040136 |
|
| [6] |
(a)
doi: 10.1039/C6RA19741H |
|
(b)
doi: 10.1039/C9GC04446A |
|
|
(c)
doi: 10.1039/C7CC03754F |
|
|
(d)
doi: 10.1246/cl.2012.728 |
|
|
(e)
doi: 10.1021/np060395l |
|
|
(f)
doi: 10.1021/ja00158a048 |
|
| [7] |
(a)
doi: 10.1021/acs.joc.1c02753 |
|
(b)
doi: 10.1039/D1CC06492D |
|
|
(c)
doi: 10.1039/D0CC06418A |
|
|
(d)
doi: 10.1039/D1GC00138H |
|
|
(e)
doi: 10.1021/acs.joc.1c01485 |
|
|
(f)
doi: 10.1021/acs.orglett.1c02808 |
|
|
(g)
doi: 10.1039/C9GC02370D |
|
|
(h)
|
|
|
(i)
doi: 10.1039/D4RA01807A |
|
|
(j)
doi: 10.6023/cjoc202407039 |
|
|
(乔秀秀, 李倩, 赵世娜, 魏瑞琪, 马桃, 何永辉, 赵晓静, 有机化学, 2025, 45, 1166.)
doi: 10.6023/cjoc202407039 |
|
|
(k)
doi: 10.6023/cjoc202405016 |
|
|
(刘玉英, 符展雄, 杨超, 罗木鹏, 班树荣, 王守国, 有机化学, 2024, 44, 3505.)
doi: 10.6023/cjoc202405016 |
|
|
(l)
doi: 10.6023/cjoc202201043 |
|
|
(庞丽萍, 杨昌杰, 林洪敏, 李心宇, 唐海涛, 潘英明, 有机化学, 2022, 42, 2117.)
doi: 10.6023/cjoc202201043 |
|
| [8] |
(a)
doi: 10.1021/ol501514b |
|
(b)
doi: 10.1021/ja401386z |
|
|
(c)
doi: 10.1021/acscatal.6b01110 |
|
|
(d)
doi: 10.1021/acs.joc.7b01714 |
|
| [9] |
(a)
doi: 10.1039/D2NJ01992B |
|
(b)
doi: 10.1002/adsc.v364.4 |
|
| [10] |
doi: 10.1039/C7OB01337J |
| [11] |
(a)
doi: 10.1039/D0QO00799D |
|
(b)
doi: 10.1002/adsc.v361.1 |
|
|
(c)
doi: 10.1002/adsc.v359.23 |
|
|
(d)
doi: 10.1021/acs.joc.1c01058 |
|
|
(e)
|
|
| [12] |
(a)
|
|
(b)
doi: 10.1021/acs.orglett.9b01823 |
|
|
(c)
doi: 10.1039/c3cc44215b |
|
| [13] |
doi: 10.1021/acs.orglett.0c02323 |
| [14] |
doi: 10.1039/C8OB03057J |
| [15] |
doi: 10.3390/molecules25020419 |
| [16] |
|
| [17] |
|
| [18] |
(a)
doi: 10.1002/adsc.v364.14 |
|
(b)
doi: 10.1021/acs.joc.2c02434 |
|
|
(c)
doi: 10.1016/j.ejmech.2014.03.059 |
|
| [19] |
doi: 10.1021/acs.orglett.0c02180 |
| [20] |
doi: 10.1021/acs.orglett.2c00085 |
| [21] |
|
| [22] |
doi: 10.1002/adsc.v353.4 |
| [23] |
doi: 10.3390/molecules25020419 |
| [24] |
|
| [25] |
(a)
doi: 10.1039/C9RA04741G pmid: 26986450 |
|
(b)
doi: 10.1021/acs.orglett.6b00244 pmid: 26986450 |
|
|
(c)
doi: 10.1021/acscatal.6b01969 pmid: 26986450 |
| [1] | 乔秀秀, 李倩, 赵世娜, 魏瑞琪, 马桃, 何永辉, 赵晓静. 2-取代的3H-吲哚-3-酮类化合物参与的C2位手性吲哚啉-3-酮类化合物的不对称合成研究进展[J]. 有机化学, 2025, 45(4): 1166-1177. |
| [2] | 解海, 张雅丽, 秦秀婷, 谷永新. 利用串联的Staudinger/aza-Wittig/芳构化反应合成1,2,4-三取代咪唑衍生物[J]. 有机化学, 2024, 44(2): 525-532. |
| [3] | 廖楚婕, 阮洪瑶, 姜峻峰, 罗伦, 胡扬根. 3-芳基-2-亚胺-苯并[e]-1,3-噁嗪-4-醇衍生物的合成及活性评价[J]. 有机化学, 2023, 43(2): 763-770. |
| [4] | 赵雪纯, 樊辉, 徐瑶, 廖小铭, 张小祥. PPh3-促进邻炔基硝基苯合成3-羟基-2-吲哚酮[J]. 有机化学, 2023, 43(11): 3997-4002. |
| [5] | 赵龙, 阳茂林, 陈皓冉, 丁明武. 三组分一锅法合成3,4-二氢喹唑啉衍生物[J]. 有机化学, 2022, 42(11): 3740-3746. |
| [6] | 刘金妮, 谢益碧, 阳青青, 黄年玉, 王龙. 基于原位捕获胺的Ugi四组分反应及其后修饰串联环化反应:“一锅法”合成六元、七元杂环化合物[J]. 有机化学, 2021, 41(6): 2374-2383. |
| [7] | 史鸿燕, 钟莹, 赵志刚. 以森田-贝里斯-希尔曼(MBH)碳酸酯为原料合成多取代1,4-二氢喹啉类衍生物[J]. 有机化学, 2021, 41(2): 677-687. |
| [8] | 蔡卫, 黄有. 有机膦氧化还原催化反应研究进展[J]. 有机化学, 2021, 41(10): 3903-3913. |
| [9] | 张静静, 姚明, 李立, 桑大永, 熊航行, 刘生鹏. 白藜芦醇、白皮杉醇和赤松素的合成[J]. 有机化学, 2020, 40(4): 1062-1067. |
| [10] | 武静, 孔晗晗, 丁明武. 12H,14H-喹唑啉并[3,4-a]-3,1-苯并噁嗪的合成[J]. 有机化学, 2016, 36(7): 1662-1667. |
| [11] | 邹雯, 贺峥嵘, 贺峥杰. 一种简便合成多取代呋喃的新方法[J]. 有机化学, 2015, 35(8): 1739-1745. |
| [12] | 王红梅, 郭树兵, 胡扬根, 曾小华, 杨光义. 新型5,6,7,8-四氢苯并噻吩并嘧啶酮衍生物的合成与抗肿瘤活性研究[J]. 有机化学, 2015, 35(5): 1075-1080. |
| [13] | 王雅珍, 朱星星, 林伟, 郑纯智, 张继振. 3-亚甲基异苯并呋喃-1(3H)-酮及其衍生物合成新方法[J]. 有机化学, 2015, 35(11): 2412-2419. |
| [14] | 郑纯智, 朱星星, 赵德建, 贾洪斌, 张继振. 3-亚烷基(亚芳基)异苯并呋喃-1(3H)-酮及其衍生物的合成研究[J]. 有机化学, 2014, 34(9): 1881-1888. |
| [15] | 杨晓梅, 张玉顺, 姚赟, 陶云海. 桃小食心虫性信息素——(Z)-7-二十碳烯-11-酮的简便合成[J]. 有机化学, 2014, 34(7): 1458-1461. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||