有机化学 ›› 2011, Vol. 31 ›› Issue (12): 2088-2094. 上一篇 下一篇
研究论文
姚春凤1,2,吕梅香3,胡雯艳4,曾和平*,1
Yao Chunfeng1,2 Lü Meixiang3 Hu Wen-yan4 Zeng Heping*,1
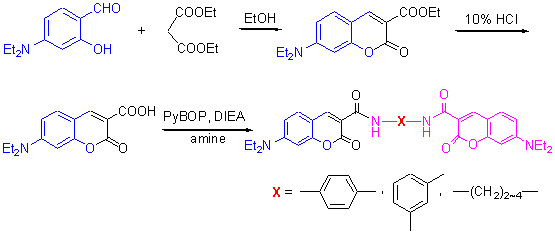
设计合成了5个新的双香豆素-3-酰胺衍生物, 并用IR, NMR, MS和元素分析进行结构表征. 用X射线单晶衍射法测定了N,N-1,3-苯基二-(7-二乙氨基-3-甲酰胺)香豆素(4)的晶体结构, 化合物4属于三斜晶系, P-1空间群, 晶胞参数a=0.8269(14) nm, b=1.4301(3) nm, c=2.5533(4) nm, α=82.55(4)°, β=86.09(5)°, γ=79.05(1)°, V=2.9367(3) nm3, Dc=1.345 g•cm-3, Z=2, F(000)=1256, μ=0.093 mm-1, 最终偏离因子R1=0.0622, wR2=0.1410. 研究了它们的紫外和荧光性质, 结果表明: 紫外吸收光谱均呈双峰结构, 化合物最大吸收波长在418~441 nm的可见光区域, 化合物的荧光发射峰在450~460 nm之间|化合物具有高的荧光量子产率, 荧光量子产率为0.56~0.65.