有机化学 ›› 2012, Vol. 32 ›› Issue (12): 2334-2338.DOI: 10.6023/cjoc201207030 上一篇 下一篇
研究简报
戢得蓉a, 粟立丹a, 张成刚a,b
Ji Deronga, Su Lidana, Zhang Chengganga,b
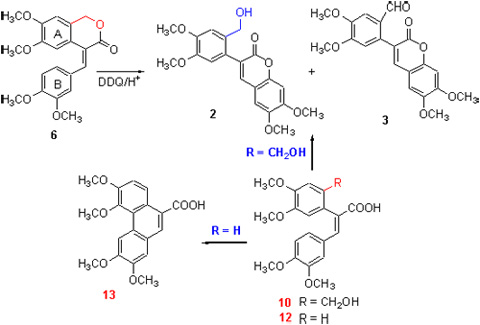
多甲氧基菲-9-甲酸及酯是合成娃儿藤生物碱及其类似物的关键中间体. 以4-(3,4-二甲氧基苯亚甲基)-6,7-二甲氧基-3-异色酮(6)为底物, 2,3-二氯-5,6-二氰基苯醌(DDQ)/CH3SO3H作为氧化体系, 没有得到预期的多甲氧基菲-9-甲酸内酯(1), 意外产物经核磁共振等确定为两个新的3-取代苯基香豆素2, 3. 进一步的实验研究显示: 底物发生分子内脱氢偶联为香豆素2而非菲环化合物1, 是因为异色酮6苯环A上2位取代基的存在所致, 其内酯环开环化合物10以及2,3-二苯基丙烯酸(12)的对比实验印证了该取代基对脱氢偶联反应选择性的影响; 异色酮6氧化偶联为香豆素2的反应机理可能为酸解开环以及途经自由基正离子的脱氢偶联, 香豆素3为DDQ氧化2的前体化合物8所得.