| [1] (a) Lin, C. M.; Singh, S. B.; Chu, P. S.; Dempcy, R. O.; Schmidt, J. M.; Pettit, G. R.; Hamel, E. Mol. Pharmacol. 1988, 34, 200.
(b) Liou, J.-P.; Chang, Y.-L.; Kuo, F. M.; Chang, C. W.; Tseng, H. Y.; Wang, C. C.; Yang, Y. N.; Chang, J. Y.; Lee, S. S.; Hsieh, H. P. J. Med. Chem. 2004, 47, 4247.
(c) Li, H.-X.; Liu, X.-R.; Zhang, W.-P.; Zhang, C.; Sun, H.-B. Chin. J. New. Drugs Clin. Rem. 2010, 29(11), 816 (in Chinese). (李洪雪, 刘晓蓉, 张文萍, 张仓, 孙宏斌, 中国新药与临床杂志, 2010, 29(11), 816.)
[2] (a) MacDonough, M. T.; Strecker, T. E.; Hamel, E.; Hall, J. J.; Chaplin, D. J.; Trawick, M. L.; Pinney, K. G. Bioorg. Med. Chem. 2013, 21, 6831.
(b) Hadimani, M. B.; MacDonough, M. T.; Ghatak, A.; Strecker, T. E.; Lopez, R.; Sriram, M.; Nguyen, B. L.; Hall, J. J.; Kessler, R. J.; Shirali, A. R.; Liu, L.; Garner, C. M.; Pettit, G. R.; Hamel, E.; Chaplin, D. J.; Mason, R. P.; Trawick, M. L.; Pinney, K. G J. Nat. Prod. 2013, 76, 1668.
(c) Flynn, B. L.; Hamel, E. WO 2002060872, 2002 [Chem. Abstr. 2002, 137, 154849].
[3] Hadimani, M.; Mejia, M.; Pinney, K.; Wang, F. US 20070082872, 2007 [Chem. Abstr. 2007, 146, 395269].
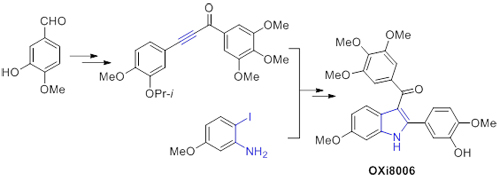
[4] Flynn, B. L.; Hamel, E.; Jung, M. K. J. Med. Chem. 2002, 45, 2670.
[5] Yuan, H.; Bi, K.-J.; Li, B.; Yue, R. C.; Ye, J.; Shen, Y. H.; Shan, L.; Jin, H. Z.; Sun, Q. Y.; Zhang, W. D. Org. Lett. 2013, 15, 4742.
[6] Beugelmans, R.; Roussi, G.; Zamora, E. G.; Carbonelle, A. C. Tetrahedron 1999, 55, 5089.
[7] Banwell, M. G.; Flynn, B. L. WO 9850365, 1998 [Chem. Abstr. 1998, 130, 3974]
[8] (a) Ridley, C. P.; Reddy, M.; Rocha, G.; Bushman, F. D.; Faulkner, D. J. Bioorg. Med. Chem. 2002, 10, 3285.
(b) Rosiak, A.; Frey, W.; Christoffers, J. Eur. J. Org. Chem. 2006, 17, 4044.
[9] (a) Kerr, D. J.; Hamel, E.; Jung, M. K.; Flynn, B. L. Bioorg. Med. Chem. 2007, 15, 3290.
(b) Yang, X.-M.; Zhang, Y.-S.; Yao, Y.; Tao, Y.-H. Chin. J. Org. Chem. 2014, 34, 1458 (in Chinese). (杨晓梅, 张玉顺, 姚赟, 陶云海, 有机化学, 2014, 34, 1458.)
[10] (a) Sakamoto, T.; Nagano, T.; Kondo, Y.; Yamanaka, H. Synthesis 1990, 215.
(b) Cai, S.; Yang, K.; Wang, D. Z. Org. Lett. 2014, 16, 2606.
(c) Würtz, S.; Rakshit, S.; Neumann, J. J.; Dröge, T.; Glorius, F. Angew. Chem., Int. Ed. 2008, 47, 7230.
(d) Batail, N.; Dufaud, V.; Djakovitch, L. Tetrahedron 2011, 52, 1916.
[11] Flynn, B. L.; Verdier-Pinard, P.; Hamel, E. Org. Lett. 2001, 3, 651. |