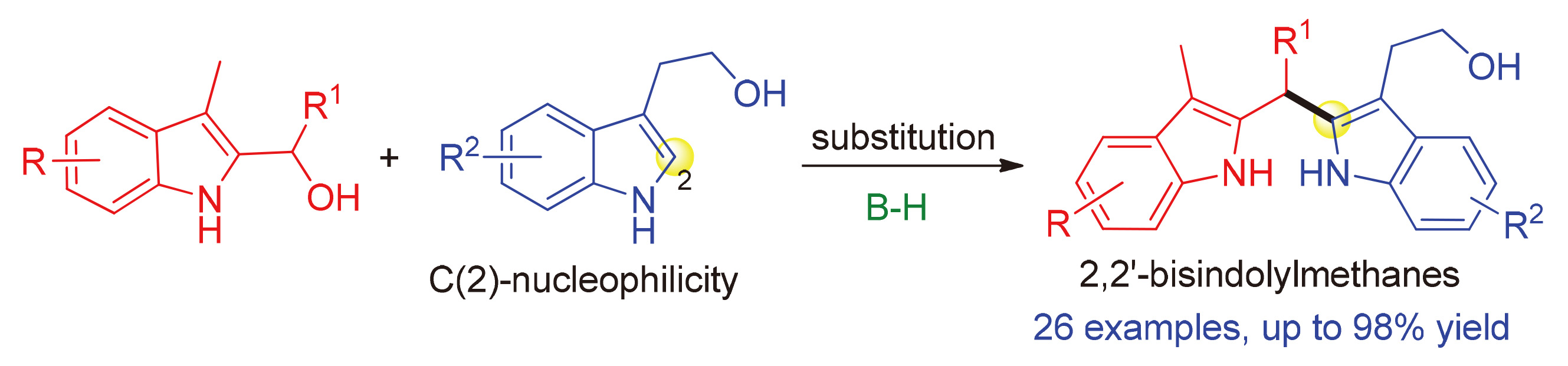
[1] For some reviews:(a) Humphrey, G. R.; Kuethe, J. T. Chem. Rev. 2006, 106, 2875.
(b) Bandini, M.; Eichholzer, A. Angew. Chem., Int. Ed. 2009, 48, 9608.
(c) Kochanowska-Karamyan, A. J.; Hamann, M. T. Chem. Rev. 2010, 110, 4489. For recent example:
(d) Pi, C.; Qu, Y.; Cui, X.; Wu, Y. Chin. J. Org. Chem. 2020, 40, 740(in Chinese). (皮超, 曲亚平, 崔秀灵, 吴养洁, 有机化学, 2020, 40, 740.)
[2] Zhao, M.; Peng, S.; Wu, J.; Wang, Y.; Gan, T. CN 106349148, 2017.
[3] Peng, S.; Zhao, M.; Wang, W.; Peng, L. WO 2014008838, 2014.
[4] Contractor, R.; Samudio, I. J.; Estrov, Z.; Harris, D.; McCubrey, J. A.; Safe, S. H.; Andreeff, M.; Konopleva, M. Cancer. Res. 2005, 65, 2890.
[5] Li, X.; Lee, S. O.; Stephen, S. Biochem. Pharmacol. 2012, 83, 1445.
[6] For some reviews:(a) Palmieri, A.; Petrini, M.; Shaikh, R. R. Org. Biomol. Chem. 2010, 8, 1259.
(b) Wang, L.; Chen, Y.; Xiao, J. Asian J. Org. Chem. 2014, 3, 1036.
(c) Mei, G.-J.; Shi, F. J. Org. Chem. 2017, 82, 7695.
(d) Zhu, S.; Xu, L.; Wang, L.; Xiao, J. Chin. J. Org. Chem. 2016, 36, 1229(in Chinese). (朱帅, 徐鲁斌, 王亮, 肖建, 有机化学, 2016, 36, 1229.)
(e) Zhang, Y.-C.; Jiang, F.; Shi, F. Acc. Chem. Res. 2020, 53, 425.
[7] For some examples on 3-indolylmethanols:(a) Sun, F.-L.; Zeng, M.; Gu, Q.; You, S.-L. Chem.-Eur. J. 2009, 15, 8709.
(b) Xiao, J. Org. Lett. 2012, 14, 1716.
(c) Wen, H.; Wang, L.; Xu, L.; Hao, Z.; Shao, C.-L.; Wang, C.-Y.; Xiao, J. Adv. Synth. Catal. 2015, 357, 4023.
(d) Xiao, J.; Wen, H.; Wang, L.; Xu, L.; Hao, Z.; Shao, C.-L.; Wang, C.-Y. Green Chem. 2016, 18, 1032.
(e) Liu, J.; Wang, L.; Wang, X.; Xu, L.; Hao, Z.; Xiao, J. Org. Biomol. Chem. 2016, 14, 11510.
(f) Xiao, M.; Ren, D.; Xu, L.; Li, S.-S.; Yu, L.; Xiao, J. Org. Lett. 2017, 19, 5724.
(g) Xu, L.; Chen, H.; Liu, J.; Zhou, L.; Liu, Q.; Lan, Y.; Xiao, J. Org. Chem. Front. 2019, 6, 1162.
[8] For substitution reactions:(a) Li, C.; Zhang, H.-H.; Fan, T.; Shen, Y.; Wu, Q.; Shi, F. Org. Biomol. Chem. 2016, 14, 6932.
(b) Zhang, H.-H.; Wang, C.-S.; Li, C.; Mei, G.-J.; Li, Y.; Shi, F. Angew. Chem., Int. Ed. 2017, 56, 116.
(c) He, Y.-Y.; Sun, X.-X.; Li, G.-H.; Mei, G.-J.; Shi, F. J. Org. Chem. 2017, 82, 2462.
(d) Zhu, Z.-Q.; Shen, Y.; Liu, J.-X.; Tao, J.-Y.; Shi, F. Org. Lett. 2017, 19, 1542.
(e) Ma, C.; Zhou, J.-Y.; Zhang, Y.-Z.; Jiao, Y.; Mei, G.-J.; Shi, F. Chem.-Asian J. 2018, 13, 2549.
(f) Xu, M.-M.; Wang, H.-Q.; Mao, Y.-J.; Mei, G.-J.; Wang, S.-L.; Shi, F. J. Org. Chem. 2018, 83, 5027.
(g) Hu, C.; Hong, G.; He, Y.; Zhou, C.; Kozlowski, M. C.; Wang, L. J. Org. Chem. 2018, 83, 4739.
(h) Zhou, Y.; Cao, W.-B.; Zhang, L.-L.; Xu, X.-P.; Ji, S.-J. J. Org. Chem. 2018, 83, 6056.
(i) Chen, L.; Zou, Y.-X.; Fang, X.-Y.; Wu, J.; Sun, X.-H. Org. Biomol. Chem. 2018, 16, 7417.
[9] For a substitution reaction:(a) Bian, C.-Y.; Li, D.; Shi, Q.; Mei, G.-J.; Shi, F. Synthesis 2018, 50, 295. For cyclization reactions:
(b) Dethe, D. H.; Boda, R.; Das, S. Chem. Commun. 2013, 49, 3260.
(c) Bera, K.; Schneider, C. Chem.-Eur. J. 2016, 22, 7074.
(d) Bera, K.; Schneider, C. Org. Lett. 2016, 18, 5660.
(e) Sun, X.-X.; Li, C.; He, Y.-Y.; Zhu, Z.-Q.; Mei, G.-J.; Shi, F. Adv. Synth. Catal. 2017, 359, 2660.
(f) Li, C.; Lu, H.; Sun, X.-X.; Mei, G.-J.; Shi, F. Org. Biomol. Chem. 2017, 15, 4794.
[10] For some reviews, see:(a) Zhuo, C.-X.; Zhang, W.; You, S.-L. Angew. Chem., Int. Ed. 2012, 51, 12662.
(b) Zhuo, C.-X.; Zheng, C.; You, S.-L. Acc. Chem. Res. 2014, 47, 2558.
(c) Liang, X.-W.; Zheng, C.; You, S.-L. Chem.-Eur. J. 2016, 22, 11918.
[11] For some examples:(a) Han, L.; Liu, C.; Zhang, W.; Shi, X.-X.; You, S.-L. Chem. Commun. 2014, 50, 1231.
(b) Liu, H.; Jiang, G.-D.; Pan, X.-X.; Wan, X.-L.; Lai, Y.-S.; Ma, D.-W.; Xie, W.-Q. Org. Lett. 2014, 16, 1908.
(c) Shao, W.; Li, H.; Liu, C.; Liu, C.-J.; You, S.-L. Angew. Chem., Int. Ed. 2015, 54, 7684.
(d) Zhang, X.; Liu, W.-B.; Tu, H.-F.; You, S.-L. Chem. Sci. 2015, 6, 4525.
(e) Ma, C.; Zhang, T.; Zhou, J.-Y.; Mei, G.-J.; Shi, F. Chem. Commun. 2017, 53, 12124.
(f) Zhang, H.-J.; Gu, Q.; You, S.-L. Org. Lett. 2019, 21, 9420.
[12] Jiang, F.; Zhao, D.; Yang, X.; Yuan, F.-R.; Mei, G.-J.; Shi, F. ACS Catal. 2017, 7, 6984.
[13] (a) Wu, J.-L.; Wang, J.-Y.; Wu, P.; Mei, G.-J.; Shi, F. Org. Chem. Front. 2017, 4, 2465.
(b) Wan, Y.; Wang, H.-Q.; Xu, M.-M.; Mei, G.-J.; Shi, F. Org. Biomol. Chem. 2018, 16, 1536.
(c) Wang, J.-Y.; Wu, P.; Wu, J.-L.; Mei, G.-J.; Shi, F. J. Org. Chem. 2018, 83, 5931.
[14] For some examples:(a) Zhang, Y.-C.; Zhao, J.-J.; Jiang, F.; Sun, S.-B.; Shi, F. Angew. Chem., Int. Ed. 2014, 53, 13912.
(b) Gong, Y.-X.; Wu, Q.; Zhang, H.-H.; Zhu, Q.-N.; Shi, F. Org. Biomol. Chem. 2015, 13, 7993.
(c) Jiang, X.; Dai, W.; Zhao, J.; Shi, F. Chin. J. Org. Chem. 2016, 36, 1014(in Chinese). (江晓莉, 戴伟, 赵佳佳, 石枫, 有机化学, 2016, 36, 1014.)
(d) Ma, C.; Jiang, F.; Sheng, F.-T.; Jiao, Y.; Mei, G.-J.; Shi, F. Angew. Chem., Int. Ed. 2019, 58, 3014.
(e) Wang, C.-S.; Li, T.-Z.; Liu, S.-J.; Zhang, Y.-C.; Deng, S.; Jiao, Y.; Shi, F. Chin. J. Chem. 2020, 38, 543.
(f) Sheng, F.-T.; Li, Z.-M.; Zhang, Y.-Z.; Sun, L.-X.; Zhang, Y.-C.; Tan, W.; Shi, F. Chin. J. Chem. 2020, 83, 583.
[15] CCDC 2010881 for 3aa. See the Supporting Information for details.
[16] For some reviews:(a) Akiyama, T. Chem. Rev. 2007, 107, 5744.
(b) Terada, M. Chem. Commun. 2008, 35, 4097.
(c) Terada, M. Synthesis 2010, 1929.
(d) Su, E.-J.; Shi, F.-J. Chin. J. Org. Chem. 2010, 30, 486(in Chinese). (苏亚军, 史福强, 有机化学, 2010, 30, 486.)
(e) Yu, J.; Shi, F.; Gong, L.-Z. Acc. Chem. Res. 2011, 44, 1156.
(f) Parmar, D.; Sugiono, E.; Raja, S.; Rueping, M. Chem. Rev. 2014, 114, 9047.
(g) Wu, H.; He, Y.-P.; Shi, F. Synthesis 2015, 47, 1990.
(h) Xia, Z.-L.; Xu-Xu, Q.-F.; Zheng, C.; You, S.-L. Chem. Soc. Rev. 2020, 49, 286. |