有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1658-1669.DOI: 10.6023/cjoc202009048 上一篇 下一篇
研究论文
收稿日期:2020-09-22
修回日期:2020-10-23
发布日期:2020-12-10
通讯作者:
雷川虎
基金资助:
Danfeng Yea,b, Hao Chenb, Zhiyuan Liub, Chuanhu Leib,*( )
)
Received:2020-09-22
Revised:2020-10-23
Published:2020-12-10
Contact:
Chuanhu Lei
About author:Supported by:文章分享

报道了一种无过渡金属和无碱条件下N-苄基-N-叔丁基羰基酰胺发生转酰胺化的方法. 该反应从高活性的N-叔丁基羰基酰亚胺出发, 历经亲核取代机理, 可以中等到优良的收率得到一系列取代酰胺产物. 值得一提的是, 由于反应体系无需添加额外的碱, 使得氨基酸衍生的手性亲核试剂及具有手性碳的酰胺在反应过程中其手性均能得到保持. 此外, 应用该方法还合成得到一些前药及抗抑郁症药物吗氯贝胺, 展示了该方法在有机合成中的潜在应用.
叶丹锋, 陈浩, 刘志园, 雷川虎. 无过渡金属及无碱参与的N-苄基-N-叔丁基羰基酰胺的转酰胺化[J]. 有机化学, 2021, 41(4): 1658-1669.
Danfeng Ye, Hao Chen, Zhiyuan Liu, Chuanhu Lei. Transamidation of N-Benzyl-N-Boc-amides under Transition Metal-Free and Base-Free Conditions[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1658-1669.
| Entry | Cs2CO3/ equiv. | Solvent | T/℃ | Conv./% of 1aa | Yielda/% of 3a |
|---|---|---|---|---|---|
| 1 | 2.0 | DMF | 100 | 41 | 33 |
| 2 | 1.0 | DMF | 100 | 70 | 48 |
| 3 | 0.2 | DMF | 100 | 85 | 63 |
| 4 | 0 | DMF | 100 | 84 | 81 |
| 5 | 0 | THF | Reflux | 97 | 83 |
| 6 | 0 | CH3CN | Reflux | 79 | 78 |
| 7 | 0 | Ether | Reflux | 93 | 81 |
| 8 | 0 | DCM | Reflux | 7 | 6 |
| 9 | 0 | Toluene | 100 | 91 | 91 |
| 10 | 0 | Toluene | 80 | 54 | 50 |
| 11 | 0 | Toluene | Reflux | 99 | 97 (90)b |
| 12c | 0 | Toluene | Reflux | 86 | 79 |
| Entry | Cs2CO3/ equiv. | Solvent | T/℃ | Conv./% of 1aa | Yielda/% of 3a |
|---|---|---|---|---|---|
| 1 | 2.0 | DMF | 100 | 41 | 33 |
| 2 | 1.0 | DMF | 100 | 70 | 48 |
| 3 | 0.2 | DMF | 100 | 85 | 63 |
| 4 | 0 | DMF | 100 | 84 | 81 |
| 5 | 0 | THF | Reflux | 97 | 83 |
| 6 | 0 | CH3CN | Reflux | 79 | 78 |
| 7 | 0 | Ether | Reflux | 93 | 81 |
| 8 | 0 | DCM | Reflux | 7 | 6 |
| 9 | 0 | Toluene | 100 | 91 | 91 |
| 10 | 0 | Toluene | 80 | 54 | 50 |
| 11 | 0 | Toluene | Reflux | 99 | 97 (90)b |
| 12c | 0 | Toluene | Reflux | 86 | 79 |
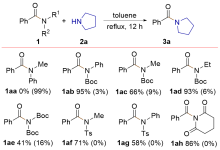




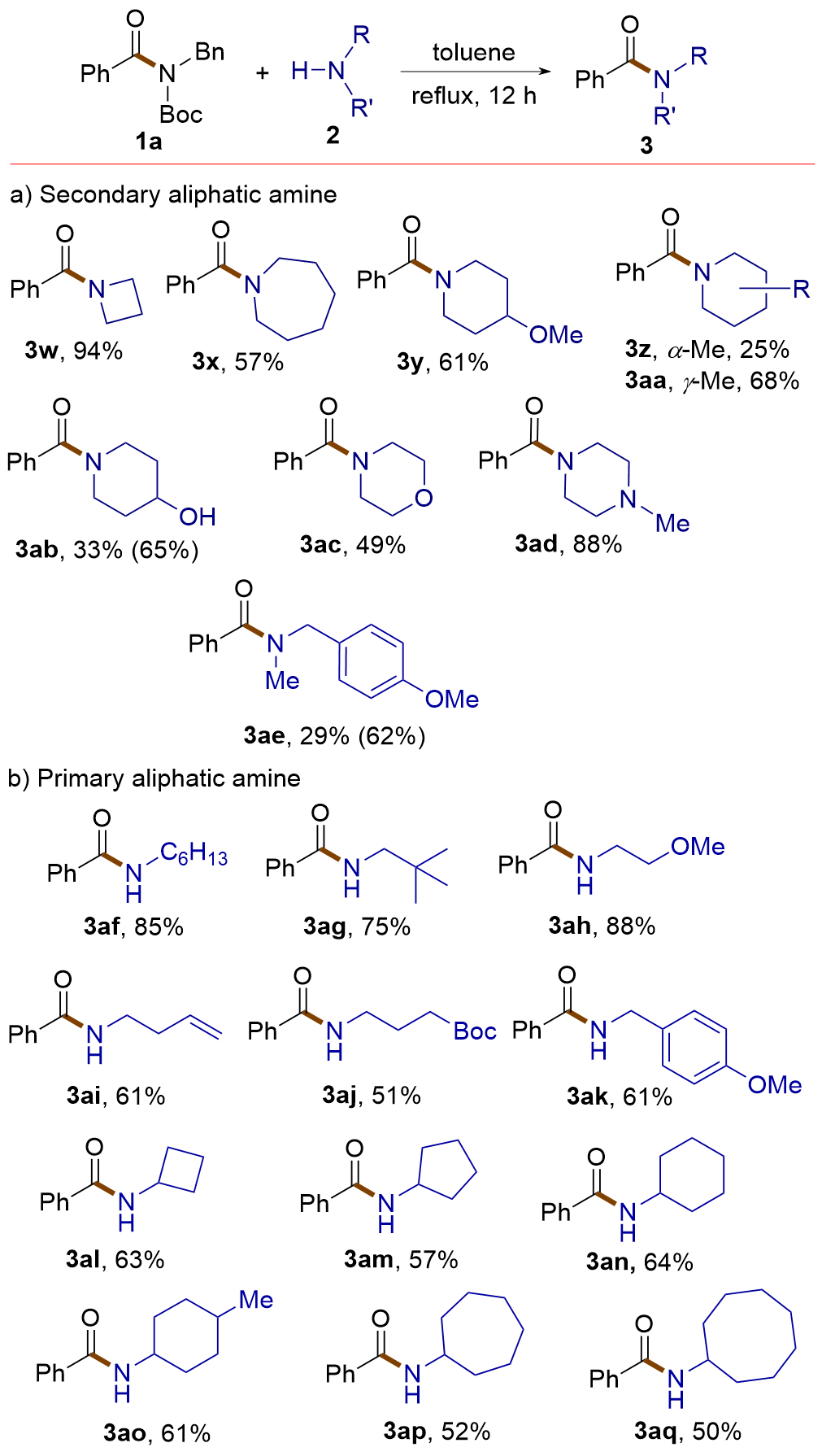
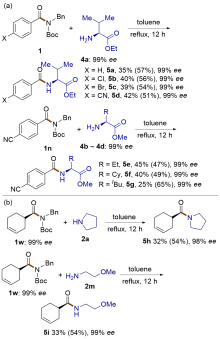
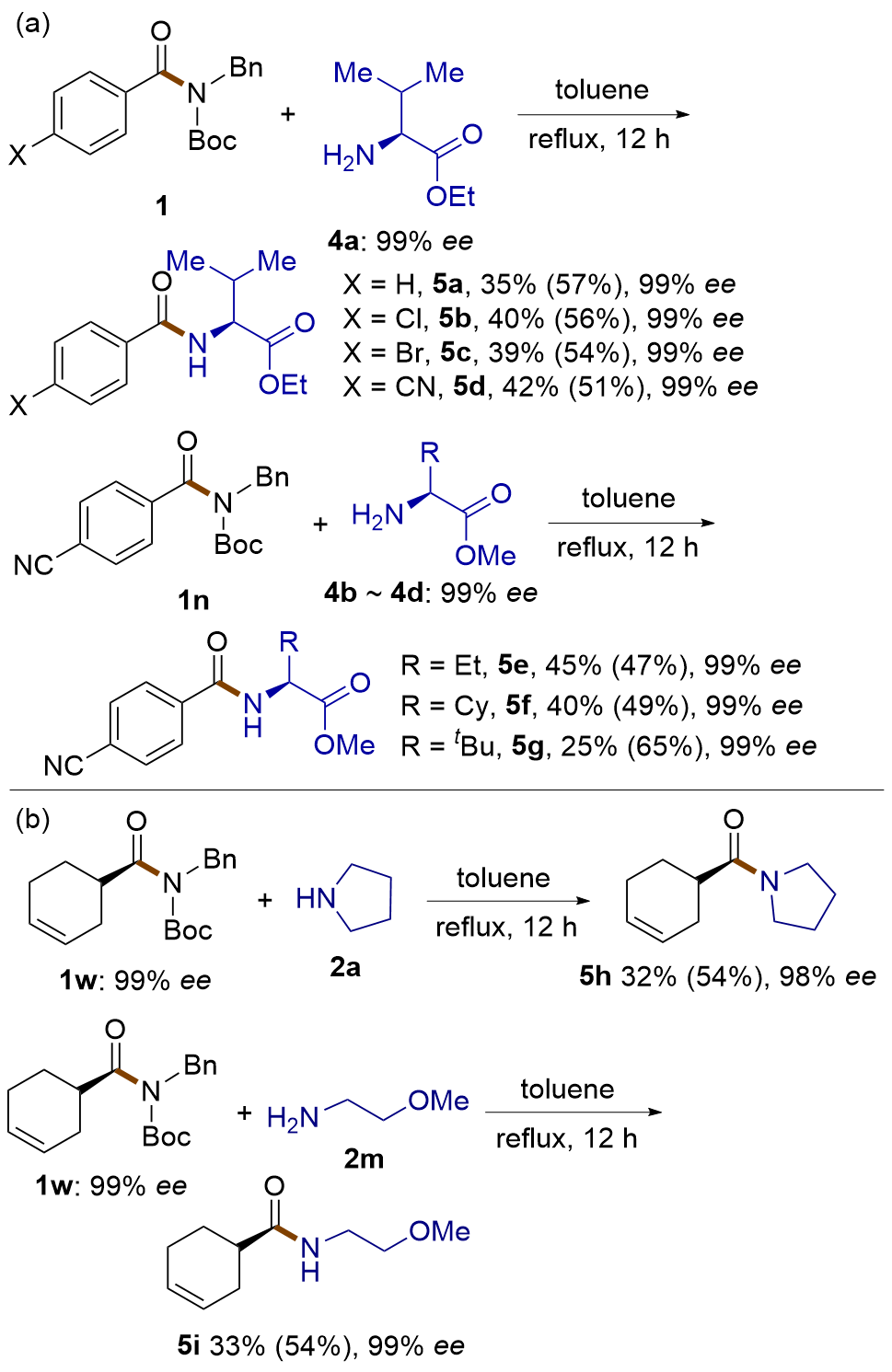



| [1] |
(a) Greenberg, A.R.; Breneman, C.M.; Liebman, J.F. The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science, Wiley-VCH, New York, 2003.
|
|
(b) Hua, X.; Liu, N.; Fan, Z.; Zong, G.; Ma, Y.; Lei, K.; Yin, H.; Wang, G. Chin. J. Org. Chem. 2019, 39,2581. (in Chinese)
|
|
|
( 华学文, 刘南南, 范志金, 宗广宁, 马翼, 雷康, 殷昊, 王桂清, 有机化学, 2019, 39,2581.)
|
|
|
(c) Zhong, L.; Jiang, T.; Zhang, F.; Fu, Q.; Liu, X.; Xu, T.; Ding, C.; Chen, J.; Yuan, J.; Tan, C. Chin. J. Org. Chem. 2019, 39,2655. (in Chinese)
|
|
|
( 钟良坤, 江涛, 张帆, 付庆, 刘幸海, 许天明, 丁成荣, 陈杰, 袁静, 谭成侠, 有机化学, 2019, 39,2655.)
|
|
| [2] |
For reviews, see: (a) Allen, C. L; Williams, J. M. J. Chem. Soc. Rev. 2011, 40,3405.
pmid: 27934465 |
|
(b) Pattabiraman, V.R.; Bode, J.W. Nature 2011, 480,471.
pmid: 27934465 |
|
|
(c) Lundberg, H.; Tinnis, F.; Selander, N.; Adolfsson, H. Chem. Soc. Rev. 2014, 43,2714.
pmid: 27934465 |
|
|
(d) Wan, J.-P.; Jing, Y. Beilstein J. Org. Chem. 2015, 11,2209.
pmid: 27934465 |
|
|
(e) de Figueiredo, R.M.; Suppo, J.-S.; Campagne, J.-M. Chem. Rev. 2016, 116,12029.
doi: 10.1021/acs.chemrev.6b00237 pmid: 27934465 |
|
|
(f) Ojeda-Porras, A.; Gamba-Sánchez, D. J. Org. Chem. 2016, 81,11548.
pmid: 27934465 |
|
|
For recent examples on the synthesis of amide, see: (g) Wang, Z.; Yang, L.; Liu, H.; Bao, W.; Tan, Y.; Wang, M.; Tang, Z.; He, W. Chin. J. Org. Chem. 2018, 38,2639. (in Chinese)
pmid: 27934465 |
|
|
( 王峥, 杨柳, 刘慧兰, 谭英芝, 包文虎, 汪明, 唐子龙, 何卫民, 有机化学, 2018, 38,2639.)
pmid: 27934465 |
|
|
(h) Xie, L.Y.; Peng, S.; Liu, F.; Yi, J.Y.; Wang, M.; Tang, Z.L.; Xu, X.H.; He, W.M. Adv. Synth. Catal. 2018, 360,4259.
pmid: 27934465 |
|
|
(i) Dissanayake, D. M., M.M.; Melville, A.D.; Vannucci, A.K. Green Chem. 2019, 21,3165.
pmid: 27934465 |
|
|
(j) Tu, Z.; Du, Y.; Cao, X.; Liu, Y. Adv. Synth. Catal. 2019, 361,4989.
pmid: 27934465 |
|
|
(k) Xie, L.-Y.; Hu, J.-L.; Song, Y.-X.; Jia, G.-K.; Lin, Y.-W.; He, J.-Y.; Cao, Z.; He, W.-M. ACS Sustainable Chem. Eng. 2019, 7,19993.
pmid: 27934465 |
|
|
(l) Yue, H.; Bao, P.; Wang, L.; Lü, X.; Yang, D.; Wang, H.; Wei, W. Chin. J. Org. Chem. 2019, 39,463. (in Chinese)
pmid: 27934465 |
|
|
( 岳会兰, 鲍鹏丽, 王雷雷, 吕晓霞, 杨道山, 王桦, 魏伟, 有机化学, 2019, 39,463.)
pmid: 27934465 |
|
|
(m) Sheng, R.; Li, P.; Zhou, Z.; Hu, G.; Zhang, X. Chin. J. Org. Chem. 2020, 40,462. (in Chinese)
pmid: 27934465 |
|
|
( 圣戎, 李萍, 周志强, 胡贵文, 张小祥, 有机化学, 2020, 40,462.)
pmid: 27934465 |
|
| [3] |
(a) Lanigan, R.M.; Sheppard, T.D. Eur. J. Org. Chem. 2013,7453.
pmid: 23473076 |
|
(b) Zheng, J.-F.; Jin, L.-R.; Huang, P.-Q. Org. Lett. 2004, 6,1139.
doi: 10.1021/ol049887k pmid: 23473076 |
|
|
(c) Tyrrell, E.; Brawn, P.; Carew, M.; Greenwood, I. Tetrahedron Lett. 2011, 52,369.
pmid: 23473076 |
|
|
(d) Allen, C.L.; Atkinson, B.N.; Williams, J.M. J. Angew. Chem. Int. Ed. 2012, 51,1383.
pmid: 23473076 |
|
|
(e) Rao, S.N.; Mohan, D.C.; Adimurthy, S. Org. Lett. 2013, 15,1496.
doi: 10.1021/ol4002625 pmid: 23473076 |
|
| [4] |
(a) Eldred, S.E.; Stone, D.A.; Gellman, S.H.; Stahl, S.S. J. Am. Chem. Soc. 2003, 125,3422.
doi: 10.1021/ja028242h pmid: 12643691 |
|
(b) Hoerter, J.M.; Otte, K.M.; Gellman, S.H.; Cui, Q.; Stahl, S.S. J. Am. Chem. Soc. 2008, 130,647.
pmid: 12643691 |
|
| [5] |
Bon, E.; Bigg, D.C. H.; Bertrand, G. J. Org. Chem. 1994, 59,4035.
|
| [6] |
(a) Gotor, V.; Brieva, R.; González, C.; Rebolledo, F. Tetrahedron 1991, 47,9207.
|
|
(b) Sergeeva, M.V.; Mozhaev, V.V.; Rich, J.O.; Khmelnitsky, Y.L. Biotechnol. Lett. 2000, 22,1419.
|
|
| [7] |
(a) Hie, L.; Fine Nathel, N.F.; Shah, T.K.; Baker, E.L.; Hong, X.; Yang, Y.-F.; Liu, P.; Houk, K.N.; Garg, N.K. Nature 2015, 524,79.
pmid: 26673267 |
|
(b) Weires, N.A.; Baker, E.L.; Garg, N.K. Nat. Chem. 2016, 8,75.
doi: 10.1038/nchem.2388 pmid: 26673267 |
|
| [8] |
(a) Baker, E.L.; Yamano, M.M.; Zhou, Y.; Anthony, S.M.; Garg, N.K. Nat. Commun. 2016, 7,11554.
doi: 10.1038/ncomms11554 pmid: 29163929 |
|
(b) Dander, J.E.; Baker, E.L.; Garg, N.K. Chem. Sci. 2017, 8,6433.
doi: 10.1039/c7sc01980g pmid: 29163929 |
|
| [9] |
(a) Meng, G.; Lei, P.; Szostak, M. Org. Lett. 2017, 19,2158.
pmid: 28397498 |
|
(b) Shi, S.; Szostak, M. Chem. Commun. 2017, 53,10584.
pmid: 28397498 |
|
| [10] |
Li, G.; Szostak, M. Chem. Rec. 2020, 20,649.
doi: 10.1002/tcr.201900072 pmid: 31833633 |
| [11] |
(a) Liu, Y.; Shi, S.; Achtenhagen, M.; Liu, R.; Szostak, M. Org. Lett. 2017, 19,1614.
doi: 10.1021/acs.orglett.7b00429 pmid: 31203613 |
|
(b) Li, G.; Szostak, M. Nat. Commun. 2018, 9,4165.
pmid: 31203613 |
|
|
(c) Liu, Y.; Achtenhagen, M.; Liu, R.; Szostak, M. Org. Biomol. Chem. 2018, 16,1322.
pmid: 31203613 |
|
|
(d) Li, G.; Ji, C.-L.; Hong, X.; Szostak, M. J. Am. Chem. Soc. 2019, 141,11161.
doi: 10.1021/jacs.9b04136 pmid: 31203613 |
|
| [12] |
Guo, W.; Huang, J.; Wu, H.; Liu, T.; Luo, Z.; Jian, J.; Zeng, Z. Org. Chem. Front. 2018, 5,2950.
|
| [13] |
Verho, O.; Pourghasemi Lati, M.; Oschmann, M. J. Org. Chem. 2018, 83,4464.
|
| [14] |
Rahman, M.M.; Li, G.; Szostak, M. J. Org. Chem. 2019, 84,12091.
pmid: 31430149 |
| [15] |
Ramkumar, R.; Chandrasekaran, S. Synthesis 2018, 51,921.
|
| [16] |
Other recent methods referring to the transamidation of amides (a) Cheung, C.W.; Ploeger, M. L.; Hu, X.; ACS Catal. 2017, 7,7092.
pmid: 32298591 |
|
(b) Cheung, C.W.; Ma, J.-A.; Hu, X. J. Am. Chem. Soc. 2018, 140,6789.
doi: 10.1021/jacs.8b03739 pmid: 32298591 |
|
|
(c) Mishra, A.; Singh, S.; Srivastava, V. Asian J. Org. Chem. 2018, 7,1600.
pmid: 32298591 |
|
|
(d) Ghosh, T.; Jana, S.; Dash, J. Org. Lett. 2019, 21,6690.
pmid: 32298591 |
|
|
(e) Sureshbabu, P.; Azeez, S.; Chaudhary, P.; Kandasamy, J. Org. Biomol. Chem. 2019, 17,845.
doi: 10.1039/c8ob03010c pmid: 32298591 |
|
|
(f) Chen, J.; Xia, Y.; Lee, S. Org. Lett. 2020, 22,3504.
doi: 10.1021/acs.orglett.0c00958 pmid: 32298591 |
|
| [17] |
Ye, D.; Liu, Z.; Chen, H.; Sessler, J.L.; Lei, C. Org. Lett. 2019, 21,6888.
|
| [18] |
(a) Galli, C. Org. Prep. Proced. Int. 1992, 24,285.
pmid: 11856006 |
|
(b) Salvatore, R.N.; Nagle, A.S.; Jung, K.W. J. Org. Chem. 2002, 67,674.
pmid: 11856006 |
|
| [19] |
(a) Meng, G.; Szostak, M. Angew. Chem., nt. Ed. 2015, 54,14518.
pmid: 27304392 |
|
(b) Meng, G.; Szostak, M. Org. Lett. 2015, 17,4364.
doi: 10.1021/acs.orglett.5b02209 pmid: 27304392 |
|
|
(c) Shi, S.; Meng, G.; Szostak, M. Angew. Chem., nt. Ed. 2016, 55,6959.
pmid: 27304392 |
|
|
(d) Shi, S.; Szostak, M. Chem.-Eur. J. 2016, 22,10420.
pmid: 27304392 |
|
| [20] |
Graton, J.; Berthelot, M.; Laurence, C. J. Chem. Soc., erkin Trans. 2 2001,2130.
|
| [21] |
The pKa of protonated N-methyl-4-methoxybenzylamine was 9.97 from website: http://ibond.nankai.edu.cn/pka/.
|
| [22] |
Bonnet, U. CNS Drug Rev. 2002, 8,283.
pmid: 12353059 |
| [23] |
Dworkin, R.H.; Kirkpatrick, P. Nat. Rev. Drug Discovery 2005, 4,455.
doi: 10.1038/nrd1756 pmid: 15959952 |
| [24] |
Sheraz, M.A.; Ahsan, S.F.; Khan, M.F.; Ahmed, S.; Ahmad, I. J. Pharm. 2016, 2016,8961621.
|
| [25] |
Bryans, J.S.; Williams, S.C.; Blakemore, D.C. EP 1178034, 2001.
|
| [26] |
(a) Yamada, S.; Hongo, C.; Yoshioka, R.; Chibata, I. J. Org. Chem. 1983, 48,843.
|
|
(b) Harry, L.G.; Pugnière, M.; Castro, B.; Previero, A. Int. J. Peptide Prorein Res. 1993, 41,323.
|
|
|
(c) Ramachandran, U.; Kumar, S.; Chawla, H.P. S. Org. Prep. Proced. Int. 2003, 35,616.
|
|
|
(d) Wang, S.M.; Zhao, C.; Zhang, X.; Qin, H.L. Org. Biomol. Chem. 2019, 17,4087.
|
|
| [27] |
(a) Cheung, C.W.; Ploeger, M.L.; Hu, X. Nat. Commun. 2017, 8,14878.
doi: 10.1038/ncomms14878 pmid: 28345585 |
|
(b) Halima, T.B.; Masson-Makdissi, J.; Newman, S.G. Angew. Chem. Int. Ed. 2018, 57,12925.
pmid: 28345585 |
|
| [28] |
(a) D, H.A.; Hang, Y. EP 20050713917, 2005.
pmid: 16634594 |
|
(b) Abergel, R.J.; Raymond, K.N. Inorg. Chem. 2006, 45,3622.
pmid: 16634594 |
|
| [29] |
Kanzian, T.; Nigst, T.A.; Maier, A.; Pichl, S.; Mayr, H. Eur. J. Org. Chem. 2009,6379.
|
| [30] |
Fox, J.M.; Dmitrenko, O.; Liao, L.-a.; Bach, R.D. J. Org. Chem. 2004, 69,7317.
doi: 10.1021/jo049494z pmid: 15471486 |
| [31] |
Adachi, S.; Kumagai, N.; Shibasaki, M. Tetrahedron Lett. 2018, 59,1147.
|
| [32] |
Kumar, V.; Connon, S.J. Chem. Commun. 2017, 53,10212.
|
| [33] |
Wang, X.-F.; Yu, S.-S.; Wang, C.; Xue, D.; Xiao, J. Org. Biomol. Chem. 2016, 14,7028.
doi: 10.1039/c6ob00736h pmid: 27363514 |
| [34] |
Chardon, A.; Mohy El Dine, T.; Legay, R.; De Paolis, M.; Rouden, J.; Blanchet, J. Chem.-Eur. J. 2017, 23,2005.
pmid: 27930832 |
| [35] |
Ning, X.-Q.; Lou, S.-J.; Mao, Y.-J.; Xu, Z.-Y.; Xu, D.-Q. Org. Lett. 2018, 20,2445.
doi: 10.1021/acs.orglett.8b00793 pmid: 29634276 |
| [36] |
Zhang, B.; Feng, P.; Cui, Y.; Jiao, N. Chem. Commun. 2012, 48,7280.
|
| [37] |
Chaudhari, M.B.; Bisht, G.S.; Kumari, P.; Gnanaprakasam, B. Org. Biomol. Chem. 2016, 14,9215.
|
| [38] |
Mondal, A.; Subaramanian, M.; Nandakumar, A.; Balaraman, E. Org. Lett. 2018, 20,3381.
doi: 10.1021/acs.orglett.8b01305 pmid: 29791162 |
| [39] |
Qian, C.; Zhang, X.; Zhang, Y.; Shen, Q. J. Organomet. Chem. 2010, 695,747.
|
| [40] |
Zhu, M.; Fujita, K.-i.; Yamaguchi, R. J. Org. Chem. 2012, 77,9102.
doi: 10.1021/jo301553v pmid: 23006061 |
| [41] |
Sawant, D.N.; Bagal, D.B.; Ogawa, S.; Selvam, K.; Saito, S. Org. Lett. 2018, 20,4397.
pmid: 30020789 |
| [42] |
Zhu, J.; Zhang, Y.; Shi, F.; Deng, Y. Tetrahedron Lett. 2012, 53,3178.
|
| [43] |
Soulard, V.; Villa, G.; Vollmar, D.P.; Renaud, P. J. Am. Chem. Soc. 2018, 140,155.
doi: 10.1021/jacs.7b12105 pmid: 29240406 |
| [44] |
Zhao, Q.; Li, H.; Wang, L. Org. Biomol. Chem. 2013, 11,6772.
pmid: 23999992 |
| [45] |
Hamada, S.; Sugimoto, K.; Iida, M.; Furuta, T. Tetrahedron Lett. 2019, 60,151277.
|
| [46] |
Rossi, S.A.; Shimkin, K.W.; Xu, Q.; Mori-Quiroz, L.M.; Watson, D.A. Org. Lett. 2013, 15,2314.
doi: 10.1021/ol401004r pmid: 23611591 |
| [47] |
Yuan, Y.-C.; Kamaraj, R.; Bruneau, C.; Labasque, T.; Roisnel, T.; Gramage-Doria, R. Org. Lett. 2017, 19,6404.
pmid: 29152976 |
| [48] |
Chernykh, A.V.; Melnykov, K.P.; Tolmacheva, N.A.; Kondratov, I.S.; Radchenko, D.S.; Daniliuc, C.G.; Volochnyuk, D.M.; Ryabukhin, S.V.; Kuchkovska, Y.O.; Grygorenko, O.O. J. Org. Chem. 2019, 84,8487.
doi: 10.1021/acs.joc.9b00719 pmid: 30990713 |
| [49] |
Tran, B.L.; Li, B.; Driess, M.; Hartwig, J.F. J. Am. Chem. Soc. 2014, 136,2555.
pmid: 24405209 |
| [50] |
Wang, Y.; Hu, X.; Morales-Rivera, C.A.; Li, G.-X.; Huang, X.; He, G.; Liu, P.; Chen, G. J. Am. Chem. Soc. 2018, 140,9678.
doi: 10.1021/jacs.8b05753 pmid: 29983059 |
| [1] | 刘杰, 韩峰, 李双艳, 陈天煜, 陈建辉, 徐清. 无过渡金属参与甲基杂环化合物与醇的选择性有氧烯基化反应[J]. 有机化学, 2024, 44(2): 573-583. |
| [2] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [3] | 徐忠荣, 万结平, 刘云云. 基于热、光以及电化学过程的无过渡金属碳-氢键硫氰化和硒氰化反应[J]. 有机化学, 2023, 43(7): 2425-2446. |
| [4] | 秦娇, 陈杰, 苏艳. 无过渡金属催化的α-溴代茚酮自由基裂解反应合成(2-氰基苯基)乙酸-2,2,6,6-四甲基哌啶酯[J]. 有机化学, 2023, 43(6): 2171-2177. |
| [5] | 马献涛, 闫晓雨, 朱影影, 牛双林, 王喻璇, 袁超. 水促进下杂芳基硫醚的绿色合成[J]. 有机化学, 2023, 43(6): 2136-2142. |
| [6] | 王睿, 高朗, 周岑, 张霄. 苯基吩噻嗪多孔有机聚合物催化的非活化末端烯烃的卤代全氟烷基化反应[J]. 有机化学, 2023, 43(3): 1136-1145. |
| [7] | 孙婧, 张萌萌, 锅小龙, 王琪, 王陆瑶. 无过渡金属条件下二芳基硒化合物的合成[J]. 有机化学, 2023, 43(12): 4251-4260. |
| [8] | 李奇阳, 张海燕, 刘文博. 无过渡金属参与的碳硅键构筑方法研究进展[J]. 有机化学, 2023, 43(10): 3470-3490. |
| [9] | 陈天煜, 韩峰, 李双艳, 刘建平, 陈建辉, 徐清. 无过渡金属参与杂环甲基化合物与醇的选择性有氧碳-烷基化反应[J]. 有机化学, 2022, 42(9): 2914-2924. |
| [10] | 许耀辉, 吴镇, 吴新鑫, 朱晨. 无过渡金属参与的醚、醛和酰胺C—H键自由基炔基化和烯丙基化反应[J]. 有机化学, 2022, 42(12): 4340-4349. |
| [11] | 王琦, 朱柏燃, 杨光, 马献涛, 徐清. 无碱条件下直接多组分反应选择性合成非对称含氮杂芳基硫醚[J]. 有机化学, 2021, 41(3): 1193-1199. |
| [12] | 刘丽, 肖洪, 肖福红, 谢艳军, 黄华文, 邓国军. 亚磺酸钠盐和芳基乙酮/茚酮合成β-酮砜[J]. 有机化学, 2021, 41(12): 4749-4757. |
| [13] | 赵保丽, 杨良凤, 程凯, 周丽云, 万结平. 无过渡金属条件下可见光诱导α-重氮酯的氧化合成α-酮酯[J]. 有机化学, 2021, 41(12): 4732-4737. |
| [14] | 张振国, 刘笑笑, 宗鑫龙, 苑亚林, 刘双磊, 张婷, 吴子尚, 杨静莹, 贾振华. 无过渡金属催化条件下合成3,3'-二吲哚甲烷衍生物的最新进展[J]. 有机化学, 2021, 41(1): 52-64. |
| [15] | 程辉成, 郭鹏虎, 陈冰, 姚嘉伟, 马姣丽, 胡炜杰, 纪红兵. 二苯并噻吩的合成研究进展[J]. 有机化学, 2021, 41(1): 94-104. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||