有机化学 ›› 2021, Vol. 41 ›› Issue (1): 52-64.DOI: 10.6023/cjoc202008003 上一篇 下一篇
所属专题: 热点论文虚拟合集
综述与进展
张振国a, 刘笑笑a, 宗鑫龙a, 苑亚林a, 刘双磊a, 张婷a, 吴子尚a, 杨静莹*( ), 贾振华a,*(
), 贾振华a,*( )
)
收稿日期:2020-08-05
修回日期:2020-10-13
发布日期:2020-10-22
通讯作者:
杨静莹, 贾振华
作者简介:基金资助:
Zhenguo Zhanga, Xiaoxiao Liua, Xinlong Zonga, Yalin Yuana, Shuanglei Liua, Ting Zhanga, Zishang Wua, Jingying Yang*( ), Zhenhua Jiaa,*(
), Zhenhua Jiaa,*( )
)
Received:2020-08-05
Revised:2020-10-13
Published:2020-10-22
Contact:
Jingying Yang, Zhenhua Jia
Supported by:文章分享
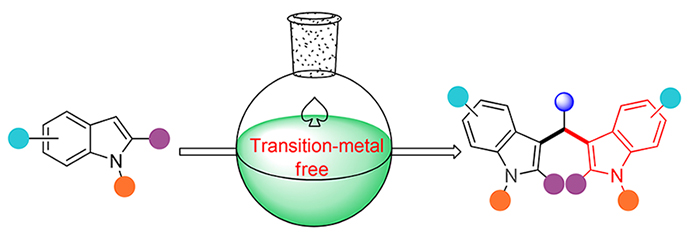
3,3'-二吲哚甲烷类化合物是一类重要的吲哚生物碱, 其结构单元广泛存在于天然产物、功能性材料以及合成药物分子中. 因其具有多样的生物活性和功能, 如抗氧化、抗炎症、抗血管生成、抗菌以及抗癌活性等, 构筑3,3'-二吲哚甲烷类杂环化合物备受关注. 传统的合成方法, 尤其是对称结构的3,3'-二吲哚甲烷类化合物的合成主要在化学量的Brønsted酸或Lewis酸存在下, 吲哚衍生物与羰基化合物经傅-克反应缩合得到. 而过渡金属的使用可引起化合物中金属残留以及环境污染. 总结和探讨了从2010年至今3,3'-二吲哚甲烷类化合物的合成方法, 尤其是对无过渡金属参与条件下, 对称结构的3,3'-二吲哚甲烷类化合物以及非对称结构3,3'-二吲哚甲烷类化合物制备的最新进展以及相应的反应机理, 旨在为该类化合物生物活性测试提供重要的理论依据和技术支持.
张振国, 刘笑笑, 宗鑫龙, 苑亚林, 刘双磊, 张婷, 吴子尚, 杨静莹, 贾振华. 无过渡金属催化条件下合成3,3'-二吲哚甲烷衍生物的最新进展[J]. 有机化学, 2021, 41(1): 52-64.
Zhenguo Zhang, Xiaoxiao Liu, Xinlong Zong, Yalin Yuan, Shuanglei Liu, Ting Zhang, Zishang Wu, Jingying Yang, Zhenhua Jia. Recent Advance on the Synthesis of 3,3'-Bisindolylmethane Derivatives under Transition-Metal-Free Catalytic Conditions[J]. Chinese Journal of Organic Chemistry, 2021, 41(1): 52-64.
| [1] |
Katritzky A.R.; Ramsden C.A.; Scriven E.F.V.; Taylor R.J.K. Comprehensive Heterocyclic Chemistry III, Elsevier, Oxford , 2008.
|
| [2] |
Irie T.; Kubushirs K.; Suzuki K.; Tsukazaki K.; Umezawa K.; Nozawa S. Anticancer Res. 1999, 31, 3061.
|
| [3] |
Hong C.; Firestone G.L.; Bjeldanes L.F. Biochem. Pharmacol. 2002, 63, 1085.
|
| [4] |
Porszasz J.; Gibiszer-Porszasz K.; Foldeak S.; Matkovics B. Experientia 1965, 21, 93.
|
| [5] |
(a) Kuethe J.T. Chimia 2006, 60, 543.
|
|
(b) Foldeak S.; Czombas J.; Matkovis B. Acta Phys. Chem. 196 5, 11, 115.
|
|
| [6] |
Bifulco G.; Bruno I.; Minale L.; Riccio R.; Calignano A.; Debitus C. J. Nat. Prod. 1994, 57, 1294.
|
| [7] |
Bifulco G.; Bruno I.; Riccio R.; Lavayre J.; Bourdy G. J. Nat. Prod. 1995, 58, 1254.
|
| [8] |
Parmeggiani F.; Rué Casamajo A.; Walton C.J.W.; Galman J.L.; Turner N.J.; Chica R.A. ACS Catal. 2019, 9, 3482.
|
| [9] |
Wan Y.C.; Li Y.H.; Yan C.X.; Yan M.; Tang Z.L. Eur. J. Med. Chem. 2019, 183, 111691.
|
| [10] |
Bandari C.; Scull E.M.; Bavineni T.; Nimmo S.L.; Gardner E.D.; Bensen R.C.; Singh S. MedChemComm 2019, 10, 1465.
|
| [11] |
Wang S.; Fang K.; Dong G.; Chen S.; Liu N.; Miao Z.; Yao J.; Li J.; Zhang W.; Sheng C. J. Med. Chem. 2015, 58, 6678.
|
| [12] |
Zhang M.-Z.; Chen Q.; Yang G.-F. Eur. J. Med. Chem. 2015, 89, 421.
|
| [13] |
Tran P.H.; Nguyen X.-T.T.; Chau D.-K.N. Asian J. Org. Chem. 2018, 7, 232.
|
| [14] |
Benabadji S.H.; Wen R.; Zheng J.; Dong X.; Yuan S. Acta Pharmacol. Sin. 2004, 25, 666.
|
| [15] |
Bharate S.B.; Bharate J.B.; Khan S.I.; Tekwani B.L.; Khan I.A.; Vishwakarma R.A. Eur. J. Med. Chem. 2013, 63, 435.
|
| [16] |
Zhou J.M.; Yuan K.Y.; Lin W.Z.; Hu X.C.; Jin Q.Q.; Niu C.G. J. Shanghai JiaoTong Univ. ( Med. Sci. ) 2018, 38, 138.
|
| [17] |
Yang W.J.; Jiao R.; Liu Y.; Sun X.D.; Sang M. Herb. Med. 2019, 38, 1146.
|
| [18] |
Dong Y.X.; Lushnikova T.; Golla R.M.; Wang X.F.; Wang G.S. Bioorg. Med. Chem. 2017, 25, 864.
|
| [19] |
Cho H.J.; Seon M.R.; Lee Y.M.; Kim J.; Kim J.K.; Kim S.G.; Park J.H. J. Nutr. 2008, 138, 17.
|
| [20] |
Kunimasa K.; Kobayashi T.; Kaji K.; Ohta T. J. Nutr. 2010, 140, 1.
|
| [21] |
Xue L.; Firestone G.L.; Bjeldanes L.F. Oncogene 2005, 24, 2343.
|
| [22] |
Wang X.L.; Liu F.; Wang Q.Z.; Xu S.; Luo J.Y. J. For. Eng. 2017, 2, 0.
|
| [23] |
Fischer H.E. Chem. Ber. 1886, 19, 2988.
|
| [24] |
Fischer H.E. Justus Liebigs Ann. Chem. 1887, 242, 372.
|
| [25] |
Yadav J.S.; Reddy B.V.S.; Padmavani B.; Gupta M.K. Tetrahedron Lett. 2004, 45, 7577.
|
| [26] |
Ferrer C.; Amijs C.H.M.; Echavarren A.M. Chem.-Eur. J. 2007, 13, 1358.
|
| [27] |
Gao R.; Yi C.S. J. Org. Chem. 2010, 75, 3144.
|
| [28] |
Tsuchimoto T.; Kanbara M. Org. Lett. 2011, 13, 912.
|
| [29] |
Xia D.; Wang Y.; Du Z.T.; Zheng Q.Y.; Wang C.Y. Org. Lett. 2012, 14, 588.
|
| [30] |
Vicenzi D.; Sgarbossa P.; Biffis A.; Tubaro C.; Basato M.; Bogialli S.; Pastore P.; Venzo A. Organometallics 2013, 32, 7153.
|
| [31] |
Chen S.P.; Li Y.X.; Ni P.H.; Huang H.W.; Deng G.J. Org. Lett. 2016, 18, 5384.
|
| [32] |
Cooper L.; Alonso J.M.; Eagling L.; Newson H.; Herath S.; Cox B.; Muoz M.P. Chem.-Eur. J. 2018, 24, 6105.
|
| [33] |
Abe T.; Nakamura S.; Yanada R.; Choshi T.; Hibino S.; Ishikura M. Org. Lett. 2 013, 15, 3622.
|
| [34] |
Shiri M.; Zolfigol M.A.; Kruger H.G.; Tanbakouchian Z. Chem. Rev. 2010, 110, 2250.
|
| [35] |
Yi W.G.; Jia Z.Y.; Li N.B.; Qiu R.H.; Chen J.Y.; Xu X.H. Chin. J. Org. Chem. 2012, 32, 2390.
|
|
( 易卫国, 贾振永, 李宁波, 邱仁华, 陈锦杨, 许新华, 有机化学, 2012, 32, 2390.).
|
|
| [36] |
Gong H.-W.; Xie Z.-F. Chin. J. Org. Chem. 2012, 32, 1195.
|
|
( 宫海伟, 解正峰, 有机化学, 2012, 32, 1195.).
|
|
| [37] |
He L.; Wang X.B.; Du G.F.; Dai B.; Jian T.Y. Chin. J. Org. Chem. 2013, 33, 988.
|
|
( 何林, 王湘波, 杜广芬, 代斌, 简腾跃, 有机化学, 2013, 33, 988.).
|
|
| [38] |
Li L.L.; Ban D.M.; Fu H.; Gong W.; Chen Z.; Yin X.G. Chin. J. Synth. Chem. 2018, 26, 757.
|
| [39] |
Yang Y.S.; Cao B.X.; Li S.B.; Zhang Y.P. J. Lanzhou Univ. Technol. 2018, 44, 72.
|
| [40] |
Zhang L.Y.; Wu B.Q.; Chen Z.T.; Hu J.J.; Zeng X.F.; Zhong G.F. Chin. J. Org. Chem. 2018, 38, 2028.
|
|
( 章吕烨, 吴彬强, 陈张涛, 胡锦锦, 曾晓飞, 钟国富, 有机化学, 2018, 38, 2028.).
|
|
| [41] |
Wu P.; Wu J.L.; Wang J.Y.; Mei G.J. Chin. J. Org. Chem. 20 18, 38, 1251.
|
|
( 伍平, 吴迦勒, 王静怡, 梅光建, 有机化学, 2018, 38, 1251.).
|
|
| [42] |
Liu T.W.; Zhang T.W.; Zhang S.T.; He J.H.; Zhang Y.T. Chem. J. Chin. Univ. 2019, 40, 719.
|
|
( 刘天伟, 张苏韬, 何江华, 张越涛, 高学校化学学报, 2019, 40, 719.).
|
|
| [43] |
Zhang R.Z.; Wang G.D.; Li H.S.; Duan G.Y.; Wang K.; Xia C.C. Chin. J. Org. Chem. 2019, 39, 1429.
|
|
( 张瑞泽, 王国栋, 李洪爽, 段桂运, 王凯, 夏成才, 有机化学, 2019, 39, 1429.).
|
|
| [44] |
Mao Y.J.; Lu Y.N.; Li T.Z.; Wu Q.; Tan W.; Shi F. Chin. J. Org. Chem. 2020, 40, 3895.
|
|
( 毛雨佳, 陆一楠, 李天真, 吴琼, 谭伟, 石枫, 有机化学, 2020, 40, 3895.).
|
|
| [45] |
Huo C.D.; Sun C.G.; Wang C.; Jia X.D.; Chang W.J. ACS Sustainable Chem. Eng. 2013, 1, 549.
|
| [46] |
Taha M.; Ismail N.H.; Imran S.; Anouar E.H.; Ali M.; Jamil W.; Uddin N.; Kashif S.M. RSC Adv. 2016, 6, 3276.
|
| [47] |
Vinay K.P.; Pazhamalai A. J. Org. Chem. 2017, 82, 12328.
|
| [48] |
Ling F.; Xiao L.; Zhong W.H. Org. Biomol. Chem. 2018, 16, 9274.
|
| [49] |
Qiao C.; Liu X.F.; He L.N. Chem.-Asian J. 2018, 13, 2664.
|
| [50] |
Huo C.D.; Kang L.S.; Xu X.L.; Jia X.D.; Wang X.C.; Xie H.S.; Yuan Y. Tetrahedron Lett. 2014, 55, 954.
|
| [51] |
Liu X.L.; Ma S.; Toy P.H. Org. Lett. 2019, 21, 9212.
|
| [52] |
Halimehjani A.Z.; Barati V. ChemistrySelect 2018, 3, 3024.
|
| [53] |
Nobuta T.; Fujiya A.; Tada N.; Miura T.; Itoh A. Synlett 2012, 23, 2975.
|
| [54] |
Jadhav S.D.; Bakshi D.; Singh A. J. Org. Chem. 2015, 80, 10187.
|
| [55] |
Zheng L.W.; Gao F.; Yang C.; Gao G.L.; Zhao Y.T.; Gao Y.; Xia W.J. Org. Lett. 2017, 19, 5086.
|
| [56] |
Yang T.B.; Lu H.A.; Shu Y.X.; Ou Y.F.; Hong L.; Au C.T.; Qiu R.H. Org. Lett. 2020 22, 827.
|
| [57] |
Liang D.Q.; Huang W.Z.; Yuan L.; Ma Y.H.; Ma J.M.; Ning D.M. Catal. Commun. 2014, 55, 11.
|
| [58] |
Shi X.L.; Lin H.K.; Li P.Y.; Zhang W.Q. ChemCatChem 2014, 6, 2947.
|
| [59] |
Kuwano S.; Suzuki T.; Arai T. Heterocycles 2018, 97, 163.
|
| [60] |
Mathavan S.; Kannan K.; Yamajala B.R.D. Org. Biomol. Chem. 2019, 17, 9620.
|
| [61] |
Peng X.J.; Zen Y.; Liu Q.; Liu L.X.; Wang H.S. Org. Chem. Front. 2019, 6, 3615.
|
| [62] |
Pathak T.P.; Osiak J.G.; Vaden R.M.; Welm B.E.; Sigman M.S. Tetrahedron 2012, 26, 5203.
|
| [63] |
Sasaki S.; Ikekame Y.; Tanayama M.; Yamauchi T.; Higashiyama K. Synlett 2012, 23, 2699.
|
| [64] |
Xiao J.; Wen H.; Wang L.; Xu L.B.; Hao Z.H.; Shao C.L.; Wang C.Y. Green Chem. 2016, 18, 1032.
|
| [65] |
Pillaiyar T.; Gorska E.; Schnakenburg G.; Müller C.E. J. Org. Chem. 2018, 83, 9902.
|
| [66] |
Sun F.L.; Zheng X.J.; Gu Q.; He Q.L.; You S.L. Eur. J. Org. Chem. 2010, 47.
|
| [67] |
Zhuo M.H.; Jiang Y.J.; Fan Y.S.; Gao Y.; Liu S.; Zhang S.Q. Org. Lett. 2014, 16, 1096.
|
| [68] |
Kim Y.; Lee J.; Jung J.Y.; Kim S.G. Tetrahedron Lett. 2019, 60, 1625.
|
| [1] | 吴思敏, 唐嘉欣, 周于佳, 徐学涛, 张昊星, 王少华. 2β-Acetoxyferruginol去醋酸基骨架衍生物抑制α-葡萄糖苷酶活性研究[J]. 有机化学, 2024, 44(2): 613-621. |
| [2] | 刘杰, 韩峰, 李双艳, 陈天煜, 陈建辉, 徐清. 无过渡金属参与甲基杂环化合物与醇的选择性有氧烯基化反应[J]. 有机化学, 2024, 44(2): 573-583. |
| [3] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [4] | 马翠云, 罗海澜, 张福华, 郭丹, 陈树兴, 王飞. 3-Pyrrolyl BODIPY的绿色生物合成、光物理性质及应用研究[J]. 有机化学, 2024, 44(1): 216-223. |
| [5] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [6] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [7] | 马虎, 黄丹凤, 王克虎, 唐朵朵, 冯杨, 任园园, 王君娇, 胡雨来. 3-(三氟甲基)吡唑类化合物的合成[J]. 有机化学, 2023, 43(9): 3257-3267. |
| [8] | 李阳, 袁锦鼎, 赵頔. 低共熔溶剂1,3-二甲基脲/L-(+)-酒石酸中(E)-2-苯乙烯基喹啉-3-羧酸类衍生物的绿色合成[J]. 有机化学, 2023, 43(9): 3268-3276. |
| [9] | 陈祖佳, 宇世伟, 周永军, 李焕清, 邱琪雯, 李妙欣, 汪朝阳. BF3•OEt2作为催化剂与合成子在有机合成中的应用进展[J]. 有机化学, 2023, 43(9): 3107-3118. |
| [10] | 王灵娜, 刘晓庆, 林钢, 金泓颖, 焦民均, 刘雪粉, 罗书平. 光促进双(4-二苯甲酮)苯醚催化C(sp3)—H键活化构建C—S键[J]. 有机化学, 2023, 43(8): 2848-2854. |
| [11] | 光明甲, 姜硕, 朱宝玉, 张如松, 王鲲鹏, 王明慧, 许良忠. 新型吡咯-2-甲酸及其衍生物的设计、合成和杀虫、杀螨活性[J]. 有机化学, 2023, 43(8): 2895-2904. |
| [12] | 徐忠荣, 万结平, 刘云云. 基于热、光以及电化学过程的无过渡金属碳-氢键硫氰化和硒氰化反应[J]. 有机化学, 2023, 43(7): 2425-2446. |
| [13] | 张维舒, 聂礼飞, Khurshed Bozorov, 阿吉艾克拜尔•艾萨, 赵江瑜. 2,5-二氨基噻吩-3,4-二羧酸二乙酯衍生物的合成及抗肿瘤活性研究[J]. 有机化学, 2023, 43(7): 2543-2552. |
| [14] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [15] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||