有机化学 ›› 2021, Vol. 41 ›› Issue (10): 3965-3982.DOI: 10.6023/cjoc202104008 上一篇 下一篇
所属专题: 南开大学化学学科创立100周年
综述与进展
收稿日期:2021-04-04
修回日期:2021-05-03
发布日期:2021-06-02
通讯作者:
苗志伟
基金资助:
Yitong Liua, Xiyuan Zhanga, Zhiwei Miaoa,b( )
)
Received:2021-04-04
Revised:2021-05-03
Published:2021-06-02
Contact:
Zhiwei Miao
Supported by:文章分享

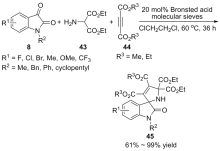
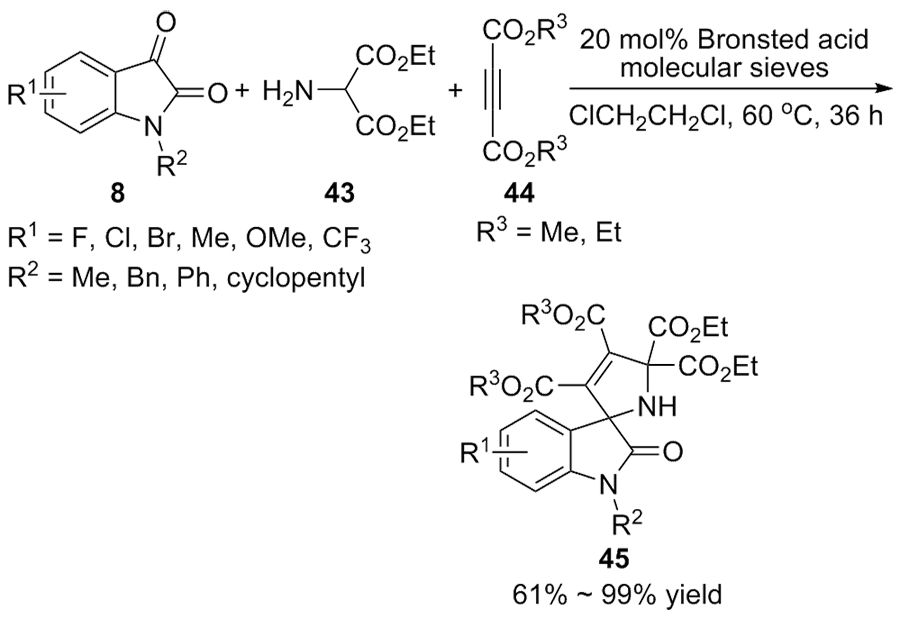
作为多种药物和天然产物的核心骨架结构, C-3位五元螺环吲哚酮衍生物在生物医药领域具有广泛的应用前景, 实现该类化合物的高效不对称合成一直是有机化学家的研究热点. 综述了近年来利用催化分子内反应、分子间反应和三组分反应不对称合成C-3位五元螺环吲哚酮衍生物的研究进展, 并对该领域的未来发展方向进行了展望.
刘奕彤, 张茜苑, 苗志伟. C-3位五元螺环吲哚酮衍生物合成方法研究进展[J]. 有机化学, 2021, 41(10): 3965-3982.
Yitong Liu, Xiyuan Zhang, Zhiwei Miao. Research Progress on the Synthetic Method of Five-Membered Spirooxindole Derivatives at C-3 Position[J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 3965-3982.

| [1] |
Mei, G.-J.; Shi, F. Chem. Commun. 2018, 54, 6607.
doi: 10.1039/C8CC02364F |
| [2] |
Cui, C.-B.; Kakeya, H.; Osada, H. Tetrahedron 1996, 52, 12651.
doi: 10.1016/0040-4020(96)00737-5 |
| [3] |
Cui, C.-B.; Kakeya, H.; Osada, H. J. Antibiot. 1996, 49, 832.
pmid: 8823522 |
| [4] |
Watson, A. F.; Liu, J.-F.; Bennaceur, K.; Drummond, C. J.; Endicott, J. A.; Golding, B. T.; Griffin, R. J.; Haggerty, K.; Lu, X.-H.; McDonnell, J. M.; Newell, D. R.; Noble, M. E. M.; Revill, C. H.; Riedinger, C.; Xu, Q.; Zhao, Y.; Lunec, J.; Hardcastle, L. R. Bioorg. Med. Chem. Lett. 2011, 21, 5916.
doi: 10.1016/j.bmcl.2011.07.084 pmid: 21875801 |
| [5] |
Zhou, J.-Y.; Zhou, S.-W. J. Ethnopharmacol. 2010, 132, 15.
doi: 10.1016/j.jep.2010.08.041 |
| [6] |
Xiao, Y.-L.; Zhou, Y.; Wang, J.; Wang, J.-X.; Liu, H. Chin. J. Org. Chem. 2015, 35, 2035. (in Chinese)
doi: 10.6023/cjoc201504045 |
|
(肖永龙, 周宇, 王江, 王进欣, 柳红, 有机化学, 2015, 35, 2035.)
doi: 10.6023/cjoc201504045 |
|
| [7] |
Ohmatsu, K.; Ando, Y.; Nakashima, T.; Ooi, T. Chem 2016, 1, 802.
doi: 10.1016/j.chempr.2016.10.012 |
| [8] |
Yin, B.-L.; Lai, J.-Q.; Zhang, Z.-R.; Jiang, H.-F. Adv. Synth. Catal. 2011, 353, 1961.
doi: 10.1002/adsc.v353.11/12 |
| [9] |
Yin, B.-L.; Huang, L.; Zhang, X.-Y.; Ji, F.-H.; Jiang, H.-F. J. Org. Chem. 2012, 77, 6365.
doi: 10.1021/jo301039y |
| [10] |
Yin, B.-L.; Huang, L.; Wang, X.-J.; Liu, J.-C.; Jiang, H.-F. Adv. Synth. Catal. 2013, 355, 370.
|
| [11] |
Yin, X.-P.; Zeng, X.-P.; Liu, Y.-L.; Liao, F.-M.; Yu, J.-S.; Zhou, F.; Zhou, J. Angew. Chem., Int. Ed. 2014, 53, 13740.
doi: 10.1002/anie.201407677 |
| [12] |
Nakagawa, M.; Matsuki, K.; Hasegawa, K.; Hino, T. J. Chem. Soc., Chem. Commun. 1982, 742.
|
| [13] |
Xu, J.; Liang, L.-X.; Zheng, H.-H.; Chi, Y.-R.; Tong, R.-B. Nat. Commun. 2019, 10, 4754.
doi: 10.1038/s41467-019-12768-4 |
| [14] |
Li, G.-F.; Huang, L.-W.; Xu, J.-C.; Sun, W.-S.; Xie, J.-Q.; Hong, L.; Wang, R. Adv. Synth. Catal. 2016, 358, 2873.
doi: 10.1002/adsc.201600441 |
| [15] |
Wang, D.-G.; Lu, X.-B.; Sun, S.-H.; Yu, H.-B.; Su, H.-M.; Wu, Y.-Z.; Zhong, F.-R. Eur. J. Org. Chem. 2019, 6028.
|
| [16] |
Liu, J.-C.; Peng, H.; Lu, L.; Xu, X.-B.; Jiang, H.-F.; Yin, B.-L. Org. Lett. 2016, 18, 6440.
doi: 10.1021/acs.orglett.6b03339 |
| [17] |
Liu, J.-C.; Xu, X.-B.; Li, J.-Y.; Liu, B.; Jiang, H.-F.; Yin, B.-L. Chem. Commun. 2016, 52, 9550.
doi: 10.1039/C6CC04298H |
| [18] |
Liu, J.-C.; Peng, H.; Yang, Y.-J.; Jiang, H.-F.; Yin, B.-L. J. Org. Chem. 2016, 81, 9695.
doi: 10.1021/acs.joc.6b01774 |
| [19] |
Chen, J.-Q.; Wei, Y.-L.; Xu, G.-Q.; Liang, Y.-M.; Xu, P.-F. Chem. Commun. 2016, 52, 6455.
doi: 10.1039/C6CC02007K |
| [20] |
Ratushnyy, M.; Kvasovs, N.; Sarkar, S.; Gevorgyan, V. Angew. Chem., Int. Ed. 2020, 59, 10316.
doi: 10.1002/anie.v59.26 |
| [21] |
Ying, A.-G.; Wu, C.-L.; Fu, Y.-Q.; Ren, S.-B.; Liang, H.-D. Chin. J. Org. Chem. 2012, 32, 1587. (in Chinese)
doi: 10.6023/cjoc201203009 |
|
(应安国, 武承林, 付永前, 任世斌, 梁华定, 有机化学, 2012, 32, 1587.)
doi: 10.6023/cjoc201203009 |
|
| [22] |
Sankar, M. G.; Castro, M. G.; Golz, C.; Strohmann, C.; Kumar, K.; Ihsar, M. P. S. Angew. Chem., Int. Ed. 2016, 55, 9709.
doi: 10.1002/anie.201603936 |
| [23] |
Feng, J.-X.; Huang, Y. Chem. Commun. 2019, 55, 14011.
doi: 10.1039/C9CC07346A |
| [24] |
Yu, C.-B.; Zheng, W.-P.; Zhan, J.-C.; Sun, Y.-C.; Miao, Z.-W. RSC Adv. 2014, 4, 63246.
doi: 10.1039/C4RA12997K |
| [25] |
Zhang, J.-Y.; Cheng, C.; Wang, D.; Miao, Z.-W. J. Org. Chem. 2017, 82, 10121.
doi: 10.1021/acs.joc.7b01582 |
| [26] |
Lin, Y.; Du, D.-M. Chin. J. Org. Chem. 2020, 40, 3214. (in Chinese)
doi: 10.6023/cjoc202005065 |
|
(林晔, 杜大明, 有机化学, 2020, 40, 3214.)
doi: 10.6023/cjoc202005065 |
|
| [27] |
Duan, S.-W.; Li, Y.; Liu, Y.-Y.; Zou, Y.-Q.; Shi, D.-Q.; Xiao, W.-J. Chem. Commun. 2012, 48, 5160.
doi: 10.1039/c2cc30931a |
| [28] |
Ding, L.-Z.; Zhong, T.-S.; Wu, H.; Wang, Y.-M. Eur. J. Org. Chem. 2014, 5139.
|
| [29] |
Buxton, C. S.; Blakemore, D. C.; Bower, J. F. Angew. Chem., Int. Ed. 2017, 56, 13824.
doi: 10.1002/anie.201707531 |
| [30] |
Zhou, P.-F.; Cai, Y.-F.; Lin, L.-L.; Lian, X.-J.; Xia, Y.; Liu, X.-H.; Feng, X.-M. Adv. Synth. Catal. 2015, 357, 695.
doi: 10.1002/adsc.v357.4 |
| [31] |
Tan, W.; Zhu, X.-T.; Zhang, S.; Xing, G.-J.; Zhu, R.-Y.; Shi, F. RSC Adv. 2013, 3, 10875.
doi: 10.1039/c3ra40874d |
| [32] |
Yu, B.; Sun, X.-N. Shi, X.-J.; Qi, P.-P.; Zheng, Y.-C.; Yu, D.-Q.; Liu, H.-M. Steroids 2015, 102, 92.
doi: 10.1016/j.steroids.2015.08.003 |
| [33] |
Shi, F.; Tao, Z.-L.; Luo, S.-W.; Tu, S.-J.; Gong, L.-Z. Chem.-Eur. J. 2012, 18, 6885.
doi: 10.1002/chem.201200358 |
| [34] |
Guo, C.; Song, J.; Gong, L.-Z. Org. Lett. 2013, 15, 2676.
doi: 10.1021/ol400983j |
| [35] |
Yang, J.; Liu, X.-W.; Wang, D.-D.; Tian, M.-Y.; Han, S.-N.; Feng, T.-T.; Liu, X.-L.; Mei, R.-Q.; Zhou, Y. Tetrahedron 2016, 72, 8523.
doi: 10.1016/j.tet.2016.10.050 |
| [36] |
Wu, M.-Y.; He, W.-W.; Liu, X.-Y.; Tan, B. Angew. Chem., Int. Ed. 2015, 54, 9409.
doi: 10.1002/anie.201504640 |
| [37] |
Salahi, F.; Taghizadeh, M. J.; Arvinnezhad, H.; Moemeni, M.; Jadidi, K.; Notas, B. Tetrahedron Lett. 2014, 55, 1515.
doi: 10.1016/j.tetlet.2013.11.097 |
| [38] |
Miao, Y.-H.; Hua, Y.-Z.; Wang, M.-C. Org. Biomol. Chem. 2019, 17, 7172.
doi: 10.1039/C9OB01233H |
| [1] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [2] | 于士航, 刘嘉威, 安碧玉, 边庆花, 王敏, 钟江春. 黑腹尼虎天牛接触性信息素的不对称合成[J]. 有机化学, 2024, 44(1): 301-308. |
| [3] | 关丽, 周艳艳, 毛永爆, 付恺森, 关文惠, 付义乐. 甲川链修饰菁染料的合成研究进展[J]. 有机化学, 2023, 43(8): 2682-2698. |
| [4] | 王玉超, 刘晋彪, 何智涛. 钯催化共轭二烯的不对称氢官能团化[J]. 有机化学, 2023, 43(8): 2614-2627. |
| [5] | 宋亭谕, 李冉, 黄利华, 贾世琨, 梅光建. N—N单键阻转异构体的催化不对称合成[J]. 有机化学, 2023, 43(6): 1977-1990. |
| [6] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| [7] | 蒙玲, 汪君. 硫代黄烷酮类衍生物的合成研究进展[J]. 有机化学, 2023, 43(3): 873-891. |
| [8] | 宋树勇, 徐森苗. 三氟甲基烯烃的选择性C-F键活化最新进展[J]. 有机化学, 2023, 43(2): 411-425. |
| [9] | 刘鹏, 钟富明, 廖礼豪, 谭伟强, 赵晓丹. 炔烃参与的去芳构化反应构建螺环己二烯酮类化合物的研究进展[J]. 有机化学, 2023, 43(12): 4019-4035. |
| [10] | 张怀远, 许诺, 唐蓉萍, 石星丽. 手性高价碘试剂诱导的不对称去芳构化反应研究进展[J]. 有机化学, 2023, 43(11): 3784-3805. |
| [11] | 匡鑫, 丁昌华, 吴奕晨, 王鹏. 手性烯丙基硅烷的催化对映选择性合成[J]. 有机化学, 2023, 43(10): 3367-3387. |
| [12] | 濮留洋, 李芷悦, 李利民, 马玉翠, 马民, 胡胜全, 吴正治. 秋水仙碱及其天然类似物(–)-N-乙酰秋水酚甲醚的不对称合成[J]. 有机化学, 2023, 43(1): 313-319. |
| [13] | 毛沅浩, 高延峰, 苗志伟. 过渡金属催化不对称环化反应合成七元环化合物研究进展[J]. 有机化学, 2022, 42(7): 1904-1924. |
| [14] | 龚婷婷, 陈智斌, 刘妙昌, 成江. 2-苯并呋喃-1(3H)-酮的合成研究进展[J]. 有机化学, 2022, 42(4): 1085-1100. |
| [15] | 姚婷, 李佳燕, 王佳明, 赵常贵. 氮杂环卡宾催化构筑含七元环结构的研究进展[J]. 有机化学, 2022, 42(4): 925-944. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||