有机化学 ›› 2021, Vol. 41 ›› Issue (10): 3948-3964.DOI: 10.6023/cjoc202104032 上一篇 下一篇
所属专题: 南开大学化学学科创立100周年
综述与进展
收稿日期:2021-04-16
修回日期:2021-05-12
发布日期:2021-06-17
通讯作者:
渠瑾
基金资助:Received:2021-04-16
Revised:2021-05-12
Published:2021-06-17
Contact:
Jin Qu
Supported by:文章分享

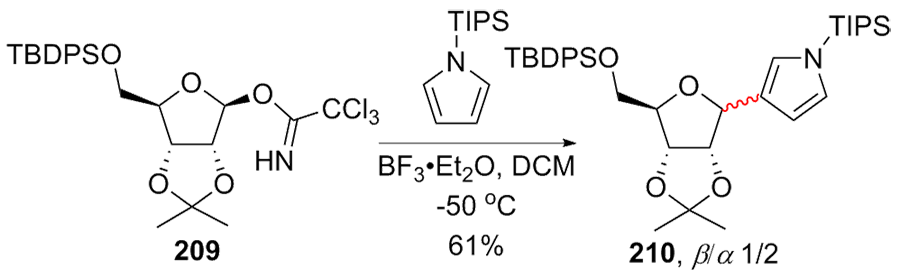
C-核苷与自然界广泛存在的N-核苷的结构高度类似, 不同之处是C-核苷中核糖与碱基是通过碳-碳键连接的. C-核苷也可以被细胞内与N-核苷相关的酶所识别和利用, 从而抑制酶促的核酸的合成或降解, 进而抑制病毒或者癌细胞的增殖, 用于治疗2019新型冠状肺炎的C-核苷药物remdesivir的临床应用引起人们对C-核苷合成的关注. 由糖基供体和糖基配体直接偶联能高效合成芳基或杂芳基C-核苷, 从核糖衍生物与有机金属试剂的反应、过渡金属催化的核糖衍生物与有机金属试剂的反应、酸催化的核糖衍生物的傅克反应三种主要合成策略总结了近年来合成芳基或杂芳基C-核苷的文献方法, 每个反应类型中又分别从半缩醛核糖、核糖内酯、卤代核糖和核糖烯等不同糖供体角度展开讨论, 并对反应中α或β构型产物的生成机理进行了详细说明.
李非凡, 渠瑾. 由糖基供体和糖基配体直接合成芳基或杂芳基C-核苷的研究进展[J]. 有机化学, 2021, 41(10): 3948-3964.
Feifan Li, Jin Qu. Synthesis of Aryl or Heteroaryl C-Nucleosides by Direct Coupling of a Carbohydrate Moiety with a Preformed Aglycon Unit[J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 3948-3964.
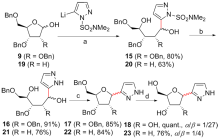
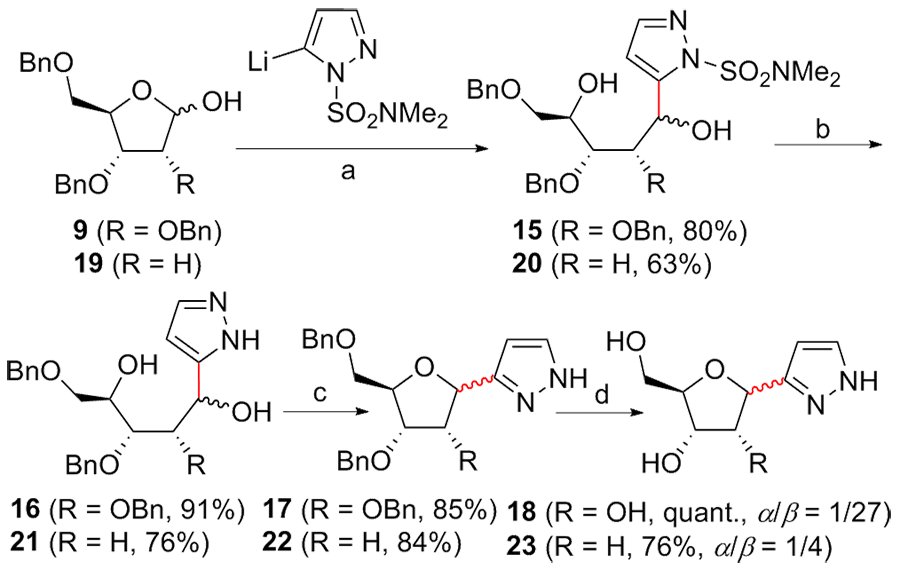
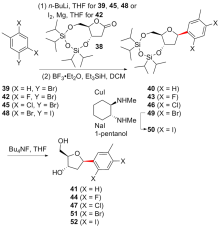

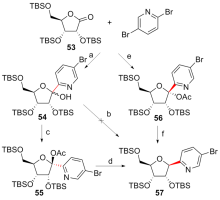
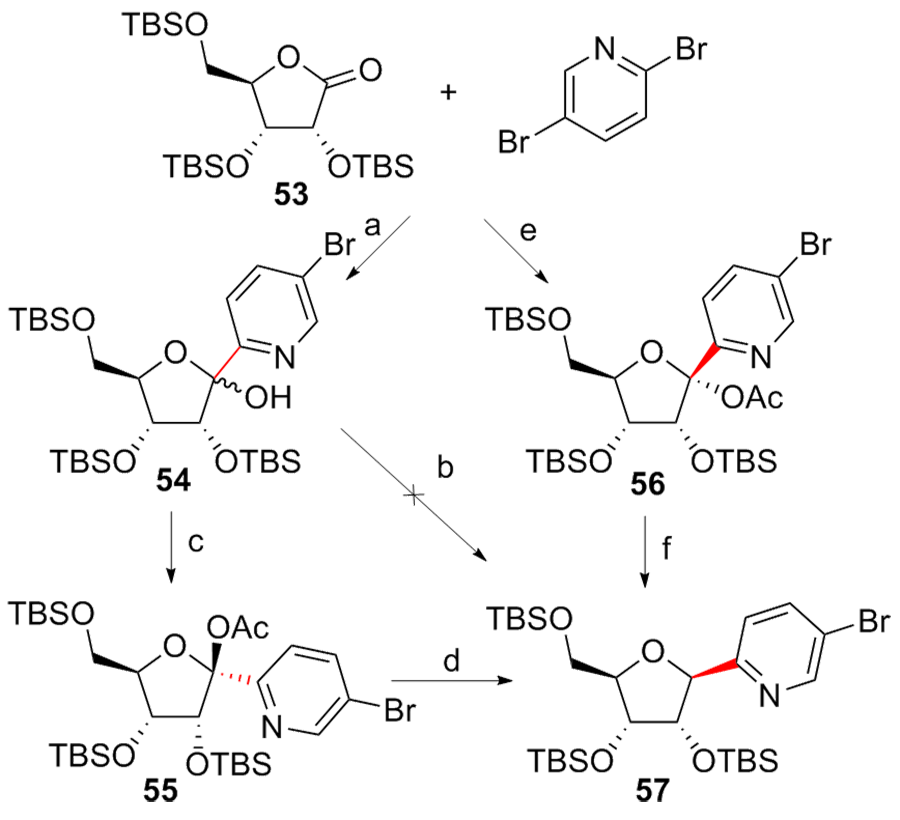
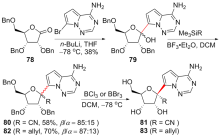

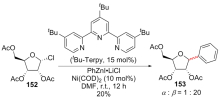
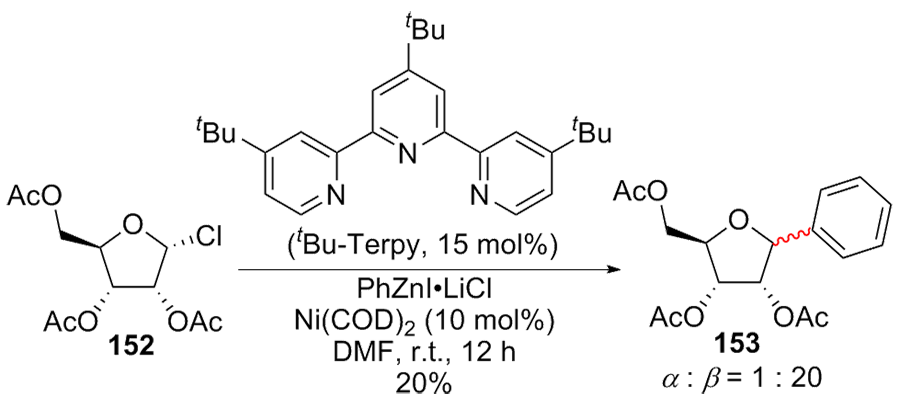
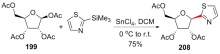
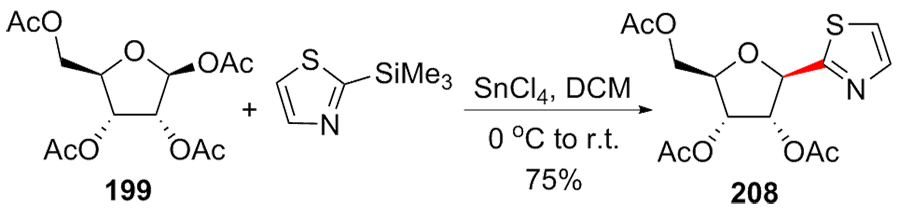
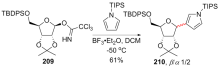
| [1] |
Štambaský, J.; Hocek, M.; Kočovský, P. Chem. Rev. 2009, 109, 6729.
doi: 10.1021/cr9002165 |
| [2] |
De Clercq, E. J. Med. Chem. 2016, 59, 2301.
doi: 10.1021/acs.jmedchem.5b01157 pmid: 26513594 |
| [3] |
Townsend, L. B. Chemistry of Nucleosides and Nucleotides, Plenum Press, New York, 1994, pp. 421-535.
|
| [4] |
(a) Arnez, J. G.; Steitz, T. A. Biochemistry 1994, 33, 7560.
pmid: 7688564 |
|
(b) Davis, D. R.; Veltri, C. A.; Nielsen, L. J. Biomol. Struct. Dyn. 1998, 15, 1121.
pmid: 7688564 |
|
|
(c) Harrington, K. M.; Nazarenko, I. A.; Dix, D. B.; Thompson, R. C.; Uhlenbeck, O. C. Biochemistry 1993, 32, 7617.
pmid: 7688564 |
|
| [5] |
Nishimura, H.; Mayama, M.; Komatsu, Y.; Shimaoka, N.; Tanaka, Y.; Kato, H. J. Antibiot. 1964, 17, 148.
|
| [6] |
Koyama, G.; Maeda, K.; Umezawa, H.; Iitaka, Y. Terahedron Lett. 1966, 7, 597.
doi: 10.1016/S0040-4039(01)99671-6 |
| [7] |
Kusakabe, Y.; Nagatsu, J.; Shibuya, M.; Kawaguchi, O.; Hirose, C.; Shirato, S. J. Antibiot. 1972, 25, 44.
pmid: 5010645 |
| [8] |
Gutowski, G. E.; Sweeney, M. J.; DeLong, D. C.; Hamill, R. L.; Gerzon, K.; Dyke, R. W. Ann. N. Y. Acad. Sci. 1975, 255, 544.
doi: 10.1111/nyas.1975.255.issue-1 |
| [9] |
(a) Robins, R. K.; Srivastava, P. C.; Narayanan, V. L.; Plowman, J.; Paull, K. D. J. Med. Chem. 1982, 25, 107.
pmid: 6130768 |
|
(b) Cooney, D. A.; Jayaram, H. N.; Gebeyehu, G.; Betts, C. R.; Kelley, J. A.; Marquez, V. E.; Johns, D. G. Biochem. Pharmacol. 1982, 31, 2133.
pmid: 6130768 |
|
|
(c) Jayaram, H. N.; Cooney, D. A.; Glazer, R. I.; Dion, R. L.; Johns, D. G. Biochem. Pharmacol. 1982, 31, 2557.
pmid: 6130768 |
|
|
(d) Jayaram, H. N.; Dion, R. L.; Glazer, R. I.; Johns, D. G.; Robins, R. K.; Srivastava, P. C.; Cooney, D. A. Biochem. Pharmacol. 1982, 31, 2371.
pmid: 6130768 |
|
|
(e) Jayaram, H. N.; Smith, A. L.; Glazer, R. I.; Johns, D. G.; Cooney, D. A. Biochem. Pharmacol. 1982, 31, 3839.
pmid: 6130768 |
|
| [10] |
Li, G.; De Clercq, E. Nat. Rev. Drug Discovery 2020, 19, 149.
doi: 10.1038/d41573-020-00016-0 |
| [11] |
Warren, T. K.; Jordan, R.; Lo, M. K.; Ray, A. S.; Mackman, R. L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H. C.; Larson, N.; Strickley, R.; Wells, J.; Stuthman, K. S.; Van Tongeren, S. A.; Garza, N. L.; Donnelly, G.; Shurtleff, A. C.; Retterer, C. J.; Gharaibeh, D.; Zamani, R.; Kenny, T.; Eaton, B. P.; Grimes, E.; Welch, L. S.; Gomba, L.; Wilhelmsen, C. L.; Nichols, D. K.; Nuss, J. E.; Nagle, E. R.; Kugelman, J. R.; Palacios, G.; Doerffler, E.; Neville, S.; Carra, E.; Clarke, M. O.; Zhang, L.; Lew, W.; Ross, B.; Wang, Q.; Chun, K.; Wolfe, L.; Babusis, D.; Park, Y.; Stray, K. M.; Trancheva, I.; Feng, J. Y.; Barauskas, O.; Xu, Y.; Wong, P.; Braun, M. R.; Flint, M.; McMullan, L. K.; Chen, S.-S.; Fearns, R.; Swaminathan, S.; Mayers, D. L.; Spiropoulou, C. F.; Lee, W. A.; Nichol, S. T.; Cihlar, T.; Bavari, S. Nature 2016, 531, 381.
doi: 10.1038/nature17180 |
| [12] |
(a) Warren, T. K.; Wells, J.; Panchal, R. G.; Stuthman, K. S.; Garza, N. L.; Van Tongeren, S. A.; Dong, L.; Retterer, C. J.; Eaton, B. P.; Pegoraro, G.; Honnold, S.; Bantia, S.; Kotian, P.; Chen, X.; Taubenheim, B. R.; Welch, L. S.; Minning, D. M.; Babu, Y. S.; Sheridan, W. P.; Bavari, S. Nature 2014, 508, 402.
doi: 10.1038/nature13027 |
|
(b) Taylor, R.; Kotian, P.; Warren, T.; Panchal, R.; Bavari, S.; Julander, J.; Dobo, S.; Rose, A.; El-Kattan, Y.; Taubenheim, B.; Babu, Y.; Sheridan, W. P. J. Infect. Public Heal. 2016, 9, 220.
|
|
| [13] |
Julander, J. G.; Bantia, S.; Taubenheim, B. R.; Minning, D. M.; Kotian, P.; Morrey, J. D.; Smee, D. F.; Sheridan, W. P.; Babu, Y. S. Antimicrob. Agents Chemother. 2014, 58, 6607.
doi: 10.1128/AAC.03368-14 pmid: 25155605 |
| [14] |
Wang, G.; Wan, J.; Hu, Y.; Wu, X.; Prhavc, M.; Dyatkina, N.; Rajwanshi, V. K.; Smith, D. B.; Jekle, A.; Kinkade, A.; Symons, J. A.; Jin, Z.; Deval, J.; Zhang, Q.; Tam, Y.; Chanda, S.; Blatt, L.; Beigelman, L. J. Med. Chem. 2016, 59, 4611.
doi: 10.1021/acs.jmedchem.5b01933 |
| [15] |
Wu, Q.; Simons, C. Synthesis 2004, 1533.
|
| [16] |
Adamo, M. F. A.; Pergoli, R. Curr. Org. Chem. 2008, 12, 1544.
doi: 10.2174/138527208786786318 |
| [17] |
Brown, D. M.; Ogden, R. C. J. Chem. Soc., Perkin Trans. 1 1981, 723.
|
| [18] |
Krohn, K.; Heins, H.; Wielckens, K. J. Med. Chem. 1992, 35, 511.
pmid: 1738143 |
| [19] |
Harusawa, S.; Matsuda, C.; Araki, L.; Kurihara, T. Synthesis 2006, 793.
|
| [20] |
Solomon, M. S.; Hopkins, P. B. Tetrahedron Lett. 1991, 32, 3297.
doi: 10.1016/S0040-4039(00)92690-X |
| [21] |
Spadafora, M.; Burger, A.; Benhida, R. Synlett 2008, 1225.
|
| [22] |
Peyron, C.; Benhida, R. Synlett 2009, 472.
|
| [23] |
Piccirilli, J. A.; Krauch, T.; Macpherson, L. J.; Benner, S. A. Helv. Chim. Acta 1991, 74, 397.
doi: 10.1002/(ISSN)1522-2675 |
| [24] |
Kim, T. W.; Kool, E. T. Org. Lett. 2004, 6, 3949.
doi: 10.1021/ol048487u |
| [25] |
van Rijssel, E. R.; van Delft, P.; Lodder, G.; Overkleeft, H. S.; van der Marel, G. A.; Filippov, D. V.; Codée, J. D. C. Angew. Chem., Int. Ed. 2014, 53, 10381.
doi: 10.1002/anie.201405477 |
| [26] |
van Rijssel, E. R.; van Delft, P.; van Marle, D. V.; Bijvoets, S. M.; Lodder, G.; Overkleeft, H. S.; van der Marel, G. A.; Filippov, D. V.; Codée, J. D. C. J. Org. Chem. 2015, 80, 4553.
doi: 10.1021/acs.joc.5b00419 pmid: 25826382 |
| [27] |
Larsen, C. H.; Ridgway, B. H.; Shaw, J. T.; Smith, D. M.; Woerpel, K. A. J. Am. Chem. Soc. 2005, 127, 10879.
doi: 10.1021/ja0524043 |
| [28] |
Štefko, M.; Slavětínská, L.; Klepetářová, B.; Hocek, M. J. Org. Chem. 2011, 76, 6619.
doi: 10.1021/jo200949c |
| [29] |
Asbun, W.; Binkley, S. B. J. Org. Chem. 1968, 31, 140.
|
| [30] |
Enders, D.; Hieronymi, A.; Ridder, A. Synlett 2005, 2391.
|
| [31] |
Guianvarc'h, D.; Fourrey, J. L.; Huu Dau, M. T.; Guérineau, V.; Benhida, R. J. Org. Chem. 2002, 67, 3724.
pmid: 12027686 |
| [32] |
Cho, A.; Saunders, O. L.; Butler, T.; Zhang, L.; Xu, J.; Vela, J. E.; Feng, J. Y.; Ray, A. S.; Kim, C. U. Bioorg. Med. Chem. Lett. 2012, 22, 2705.
doi: 10.1016/j.bmcl.2012.02.105 |
| [33] |
Metobo, S. E.; Xu, J.; Saunders, O. L.; Butler, T.; Aktoudianakis, E.; Cho, A.; Kim, C. U. Tetrahedron Lett. 2012, 53, 484.
doi: 10.1016/j.tetlet.2011.11.055 |
| [34] |
Chaudhuri, N. C.; Kool, E. T. Tetrahedron Lett. 1995, 36, 1795.
doi: 10.1016/0040-4039(95)00145-3 |
| [35] |
Shapiro, R.; Chambers, R. W. J. Am. Chem. Soc. 1961, 83, 3920.
doi: 10.1021/ja01479a057 |
| [36] |
Schweitzer, B. A.; Kool, E. T. J. Org. Chem. 1994, 59, 7238.
pmid: 20882116 |
| [37] |
Hocek, M.; Pohl, R.; Klepetářová, B. Eur. J. Org. Chem. 2005, 2005, 4525.
doi: 10.1002/(ISSN)1099-0690 |
| [38] |
Joubert, N.; Urban, M.; Pohl, R.; Hocek, M. Synthesis 2008, 1918.
|
| [39] |
Weinberger, M.; Wagenknecht, H. A. Synthesis 2012, 44, 648.
|
| [40] |
Jiang, Y. L.; Stivers, J. T. Tetrahedron Lett. 2003, 44, 4051.
doi: 10.1016/S0040-4039(03)00848-7 |
| [41] |
Nicolas, L.; Angibaud, P.; Stansfield, I.; Meerpoel, L.; Reymond, S.; Cossy, J. Tetrahedron Lett. 2014, 55, 849.
doi: 10.1016/j.tetlet.2013.12.035 |
| [42] |
Beuck, C.; Singh, I.; Bhattacharya, A.; Hecker, W.; Parmar, V. S.; Seitz, O.; Weinhold, E. Angew. Chem., Int. Ed. 2003, 42, 3958.
doi: 10.1002/(ISSN)1521-3773 |
| [43] |
Ren, R. X.-F.; Chaudhuri, N. C.; Paris, P. L.; Rumney, S.; Kool, E. T. J. Am. Chem. Soc. 1996, 118, 7671.
doi: 10.1021/ja9612763 |
| [44] |
Hainke, S.; Singh, I.; Hemmings, J.; Seitz, O. J. Org. Chem. 2007, 72, 8811.
pmid: 17941674 |
| [45] |
Singh, I.; Seitz, O. Org. Lett. 2006, 8, 4319.
doi: 10.1021/ol061701p |
| [46] |
Hacksell, U.; Daves, G. D. J. Org. Chem. 1983, 48, 2870.
doi: 10.1021/jo00165a017 |
| [47] |
Cheng, J. C. Y.; Hacksell, U.; Daves, G. D. J. Org. Chem. 1986, 51, 3093.
doi: 10.1021/jo00366a003 |
| [48] |
Zhang, H. C.; Daves, G. D. J. Org. Chem. 1992, 57, 4690.
doi: 10.1021/jo00043a029 |
| [49] |
Raboisson, P.; Baurand, A.; Cazenave, J. P.; Gachet, C.; Schultz, D.; Spiess, B.; Bourguignon, J. J. J. Org. Chem. 2002, 67, 8063.
pmid: 12423133 |
| [50] |
Lefoix, M.; Mathis, G.; Kleinmann, T.; Truffert, J. C.; Asseline, U. J. Org. Chem. 2014, 79, 3221.
doi: 10.1021/jo5000253 |
| [51] |
Joubert, N.; Pohl, R.; Klepetářová, B.; Hocek, M. J. Org. Chem. 2007, 72, 6797.
doi: 10.1021/jo0709504 |
| [52] |
Chapuis, H.; Kubelka, T.; Joubert, N.; Pohl, R.; Hocek, M. Eur. J. Org. Chem. 2012, 1759.
|
| [53] |
Temburnikar, K.; Brace, K.; Seley-Radtke, K. L. J. Org. Chem. 2013, 78, 7305.
doi: 10.1021/jo400913k pmid: 23806030 |
| [54] |
Gong, H.; Gagné, M. R. J. Am. Chem. Soc. 2008, 130, 12177.
doi: 10.1021/ja8041564 |
| [55] |
Nicolas, L.; Izquierdo, E.; Angibaud, P.; Stansfield, I.; Meerpoel, L.; Reymond, S.; Cossy, J. J. Org. Chem. 2013, 78, 11807.
doi: 10.1021/jo401845q pmid: 24127819 |
| [56] |
Adak, L.; Kawamura, S.; Toma, G.; Takenaka, T.; Isozaki, K.; Takaya, H.; Orita, A.; Li, H. C.; Shing, T. K. M.; Nakamura, M. J. Am. Chem. Soc. 2017, 139, 10693.
doi: 10.1021/jacs.7b03867 |
| [57] |
Wang, Q.; An, S.; Deng, Z.; Zhu, W.; Huang, Z.; He, G.; Chen, G. Nat. Catal. 2019, 2, 793.
doi: 10.1038/s41929-019-0324-5 |
| [58] |
Ma, Y.; Liu, S.; Xi, Y.; Li, H.; Yang, K.; Cheng, Z.; Wang, W.; Zhang, Y. Chem. Commun. 2019, 55, 14657.
doi: 10.1039/C9CC07184A |
| [59] |
Mukaiyama, T.; Matsubara, K.; Hora, M. Synthesis 1994, 1368.
|
| [60] |
Shirakawa, S.; Kobayashi, S. Org. Lett. 2007, 9, 311.
pmid: 17217292 |
| [61] |
Sokolova, T. N.; Yartseva, I. V.; Preobrazhenskaya, M. N. Carbohydr. Res. 1981, 93, 19.
doi: 10.1016/S0008-6215(00)80749-1 |
| [62] |
Hainke, S.; Arndt, S.; Seitz, O. Org. Biomol. Chem. 2005, 3, 4233.
pmid: 16294252 |
| [63] |
Bárta, J.; Pohl, R.; Klepetářová, B.; Ernsting, N. P.; Hocek, M. J. Org. Chem. 2008, 73, 3798.
doi: 10.1021/jo800177y pmid: 18416574 |
| [64] |
Macdonald, S. J. F.; Huizinga, W. B.; McKenzie, T. C. J. Org. Chem. 1988, 53, 3371.
doi: 10.1021/jo00249a051 |
| [65] |
Yokoyama, M.; Nomura, M.; Togo, H.; Seki, H. J. Chem. Soc., erkin Trans. 1 1996, 2145.
|
| [66] |
Girgis, N. S.; Michael, M. A.; Smee, D. F.; Alaghamandan, H. A.; Robins, R. K.; Cottam, H. B. J. Med. Chem. 1990, 33, 2750.
pmid: 2170645 |
| [67] |
Liu, M. C.; Luo, M. Z.; Mozdziesz, D. E.; Sartorelli, A. C. Nucleosides Nucleotides Nucleic Acids 2005, 24, 45.
doi: 10.1081/NCN-46784 |
| [68] |
Shin, D.; Sinkeldam, R. W.; Tor, Y. J. Am. Chem. Soc. 2011, 133, 14912.
doi: 10.1021/ja206095a |
| [69] |
Spadafora, M.; Mehiri, M.; Burger, A.; Benhida, R. Tetrahedron Lett. 2008, 49, 3967.
doi: 10.1016/j.tetlet.2008.04.105 |
| [70] |
Marzag, H.; Zerhouni, M.; Tachallait, H.; Demange, L.; Robert, G.; Bougrin, K.; Auberger, P.; Benhida, R. Bioorg. Med. Chem. 2018, 28, 1931.
doi: 10.1016/j.bmcl.2018.03.063 |
| [71] |
Tachallait, H.; Filho, M. S.; Marzag, H.; Bougrin, K.; Demange, L.; Martin, A. R.; Benhida, R. New J. Chem. 2019, 43, 5551.
doi: 10.1039/c8nj06300a |
| [72] |
Hungerford, N. L.; Armitt, D. J.; Banwell, M. G. Synthesis 2003, 1837.
|
| [1] | 徐伟, 翟宏斌, 程斌, 汪太民. 可见光诱导的钯催化Heck反应[J]. 有机化学, 2023, 43(9): 3035-3054. |
| [2] | 曾旭群, 张倩, 吴旭枫, 张静枫, 张鑫伟, 黄晓雷. 镍催化芳基碳酸酯和芳基氨基磺酸酯与环烯烃的Heck反应[J]. 有机化学, 2022, 42(9): 2981-2987. |
| [3] | 王春霞, 胡晓东, 许斌, 曹春阳. 靶向新型冠状病毒SARS-CoV-2主蛋白酶的抗病毒药物研究进展[J]. 有机化学, 2022, 42(7): 1974-1999. |
| [4] | 肖霄, 刘建超. 烯烃参与的还原Heck反应构建C(sp2)—C(sp3)键研究进展[J]. 有机化学, 2021, 41(9): 3349-3365. |
| [5] | 连凌翔, 尹静怡, 林彩霞, 叶克印, 袁耀锋. 膦氧导向钯催化邻碳硼烷Heck类型的官能团化反应[J]. 有机化学, 2021, 41(8): 3249-3255. |
| [6] | 曾鸿运, 张军干, 洪伟. 合成2,5-二芳基噻唑衍生物的新方法[J]. 有机化学, 2020, 40(8): 2535-2542. |
| [7] | 韩吉来, 唐美麟, 孙逊. 白藜芦醇二聚体Quadrangularin A和Pallidol合成方法学研究[J]. 有机化学, 2020, 40(6): 1571-1577. |
| [8] | Viacheslav I. Sokolov. N-环钯化二茂铁衍生物的合成和应用(英文)[J]. 有机化学, 2018, 38(1): 75-85. |
| [9] | 董旭, 侯永正, 孟凡威, 刘洪波, 刘会. 烷基Heck反应研究进展[J]. 有机化学, 2017, 37(5): 1088-1098. |
| [10] | 聂飚, 金传飞, 钟文和, 任青云, 张英俊, 张霁. 磷酰胺酯前药策略及ProTide技术在药物研发中的应用与进展[J]. 有机化学, 2017, 37(11): 2818-2840. |
| [11] | 李志伟, 康绍英, 陈琳, 王宇, 李江胜. 钯催化合成氧化芪三酚的研究[J]. 有机化学, 2016, 36(5): 1143-1147. |
| [12] | 郝二军, 蒋小涵, 付丹丹, 王东超, 谢明胜, 渠桂荣, 郭海明. 水溶液中钯催化的Heck/氰基化串联反应合成3-氰甲基吲哚酮[J]. 有机化学, 2016, 36(11): 2746-2751. |
| [13] | 尚筱洁, 柳忠全. 钯催化芳烃/烯烃与烯丙酯的氧化Heck交叉偶联反应的最新研究进展[J]. 有机化学, 2015, 35(3): 522-527. |
| [14] | 刘春廷, 毕凯健, 畅婉琳, 叶霁, 张卫东, 孙青龑. 微管蛋白聚合抑制剂OXi8006的合成新方法[J]. 有机化学, 2015, 35(2): 484-489. |
| [15] | 管成飞, 钱鹰. 含吡啶端基9,10-双芳基蒽共轭分子的合成、聚集诱导荧光增强及双光子诱导荧光[J]. 有机化学, 2014, 34(3): 537-545. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
