有机化学 ›› 2021, Vol. 41 ›› Issue (12): 4798-4807.DOI: 10.6023/cjoc202106044 上一篇 下一篇
所属专题: 绿色合成化学专辑
研究论文
王玉超a,b, 刘晋彪a,*( ), 邱观音生b,*(
), 邱观音生b,*( ), 杨宇b, 周宏伟b,*(
), 杨宇b, 周宏伟b,*( )
)
收稿日期:2021-06-24
修回日期:2021-08-22
发布日期:2021-09-02
通讯作者:
刘晋彪, 邱观音生, 周宏伟
基金资助:
Yuchao Wanga,b, Jinbiao Liua( ), Guanyinsheng Qiub(
), Guanyinsheng Qiub( ), Yu Yangb, Hongwei Zhoub(
), Yu Yangb, Hongwei Zhoub( )
)
Received:2021-06-24
Revised:2021-08-22
Published:2021-09-02
Contact:
Jinbiao Liu, Guanyinsheng Qiu, Hongwei Zhou
Supported by:文章分享
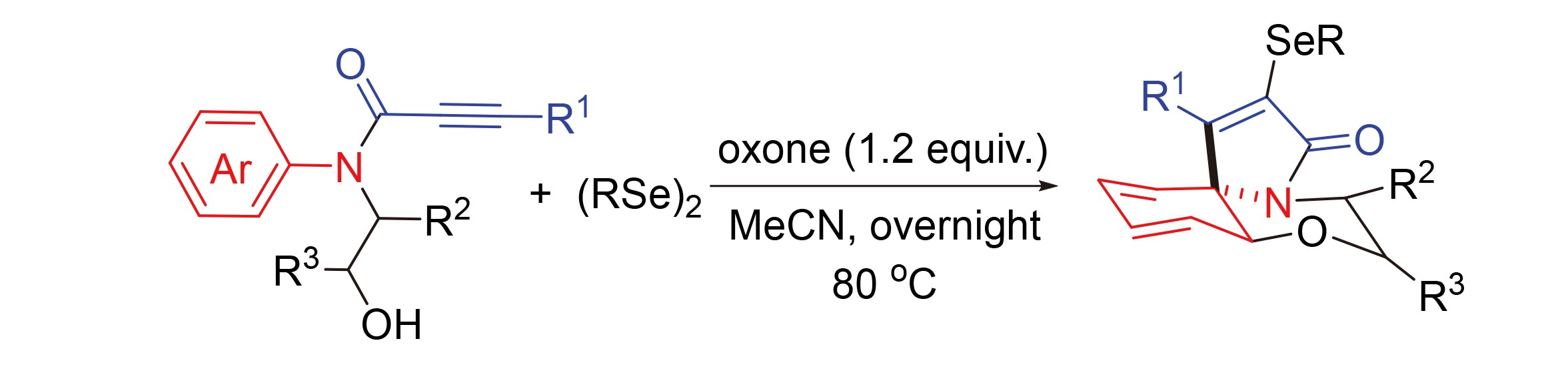
报道了一种温和的经由N-羟乙基-N-芳基丙炔酰胺的自位环化及选择性邻位捕捉的方法, 并高效合成了系列含硒苯并[b]吡咯并[2,1-c][1,4]噁嗪-3-酮化合物. 反应无需过渡金属催化, 具有较高的效率和较广的底物适应范围. 该串联环化过程包含了炔烃的α-加成、ipso-环化和螺环中间体的邻位俘获.
王玉超, 刘晋彪, 邱观音生, 杨宇, 周宏伟. N-羟乙基-N-芳基丙炔酰胺的无金属硒化螺三环化反应[J]. 有机化学, 2021, 41(12): 4798-4807.
Yuchao Wang, Jinbiao Liu, Guanyinsheng Qiu, Yu Yang, Hongwei Zhou. Metal-Free Selenizative spiro-Tricyclization of N-Hydroxylethyl-N-arylpropiolamides[J]. Chinese Journal of Organic Chemistry, 2021, 41(12): 4798-4807.
| Entry | Oxidant (equiv.) | Solvent | Temp./℃ | Yielda/% of 3a |
|---|---|---|---|---|
| 1 | Oxone (2.0) | CH3CN | 80 | 61 |
| 2 | Oxone (2.0) | DCE | 80 | 20 |
| 3 | Oxone (2.0) | THF | 80 | 17 |
| 4 | Oxone (2.0) | DMSO | 80 | 44 |
| 5 | Oxone (2.0) | 1,4-Dioxane | 80 | 13 |
| 6 | Oxone (2.0) | DMF | 80 | 42 |
| 7 | BPO (2.0) | CH3CN | 80 | 10 |
| 8 | K2S2O8 (2.0) | CH3CN | 80 | 38 |
| 9 | TBHP (2.0) | CH3CN | 80 | 0 |
| 10 | (NH4)2S2O8 (2.0) | CH3CN | 80 | Trace |
| 11 | Oxone (1.2) | CH3CN | 80 | 70 |
| 12 | Oxone (1.2) | CH3CN | r.t. | Trace |
| Entry | Oxidant (equiv.) | Solvent | Temp./℃ | Yielda/% of 3a |
|---|---|---|---|---|
| 1 | Oxone (2.0) | CH3CN | 80 | 61 |
| 2 | Oxone (2.0) | DCE | 80 | 20 |
| 3 | Oxone (2.0) | THF | 80 | 17 |
| 4 | Oxone (2.0) | DMSO | 80 | 44 |
| 5 | Oxone (2.0) | 1,4-Dioxane | 80 | 13 |
| 6 | Oxone (2.0) | DMF | 80 | 42 |
| 7 | BPO (2.0) | CH3CN | 80 | 10 |
| 8 | K2S2O8 (2.0) | CH3CN | 80 | 38 |
| 9 | TBHP (2.0) | CH3CN | 80 | 0 |
| 10 | (NH4)2S2O8 (2.0) | CH3CN | 80 | Trace |
| 11 | Oxone (1.2) | CH3CN | 80 | 70 |
| 12 | Oxone (1.2) | CH3CN | r.t. | Trace |
| [1] |
(a) Wang, Z. Org. Chem. Front. 2020, 7, 3815.
doi: 10.1039/D0QO00763C |
|
(b) Lanke, V.; Marek, I. Chem. Sci. 2020, 11, 9378.
doi: 10.1039/D0SC02562C |
|
|
(c) Li, C. X.; Ragab, S. S.; Liu, G. D.; Tang, W. J. Nat. Prod. Rep. 2020, 37, 276.
doi: 10.1039/C9NP00039A |
|
|
(d) Quiclet-Sire, B.; Zard, S. Z. Sci. China: Chem. 2019, 62, 1450.
doi: 10.1007/s11426-019-9587-x |
|
|
(e) Xu, P. W.; Yu, J. S.; Chen, C.; Cao, Z. Y.; Zhou, F.; Zhou, J. ACS Catal. 2019, 9, 1820.
doi: 10.1021/acscatal.8b03694 |
|
| [2] |
For selected reviews, see: (a) Yang, W.-C.; Zhang, M.-M.; Feng, J.-G. Adv. Synth. Catal. 2020, 362, 4446.
doi: 10.1002/adsc.v362.21 |
|
(b) Huck, C. J.; Sarlah, D. Chem. 2020, 6, 1589.
doi: 10.1016/j.chempr.2020.06.015 |
|
|
(c) Xia, Z. L.; Xu-Xu, Q.-F.; Zheng, C.; You, S.-L. Chem. Soc. Rev. 2020, 49, 286.
doi: 10.1039/C8CS00436F |
|
|
(d) Lv, S. D.; Zhang, G. F.; Chen, J. B.; Gao, W. Adv. Synth. Catal. 2020, 362, 462.
doi: 10.1002/adsc.v362.3 |
|
|
(e) Zeidan, N.; Lautens, M. Synthesis 2019, 51, 4137.
doi: 10.1055/s-0037-1611918 |
|
| [3] |
For recent examples, see: (a) Grams, R. J.; Garcia, C. J.; Szwetkoski, C.; Santos, W. L. Org. Lett. 2020, 22, 7013.
doi: 10.1021/acs.orglett.0c02567 pmid: 32610935 |
|
(b) Lu, L.; Guo, C. G.; Peng, H.; Jiang, H.; Lei, M.; Yin, B. L. Org. Lett. 2019, 21, 2602.
doi: 10.1021/acs.orglett.9b00573 pmid: 32610935 |
|
|
(c) Lee, W.; Lee, Y.; Yoo, M.; Han, S.; Kim, H. Org. Chem. Front. 2020, 7, 3209.
doi: 10.1039/D0QO00893A pmid: 32610935 |
|
|
(d) Song, L.; Tian, G.; Van, M. L.; Van der Eycken, E. V. Org. Lett. 2020, 22, 6537.
doi: 10.1021/acs.orglett.0c02310 pmid: 32610935 |
|
|
(e) Cheng, C.; Zuo, X.; Tu, D.; Wan, B.; Zhang, Y. Org. Lett, 2020, 22, 4985.
doi: 10.1021/acs.orglett.0c01513 pmid: 32610935 |
|
|
(f) Pramanik, M.; Choudhuri, K.; Chakrabory, S.; Ghosh, A.; Mal, P. Chem. Commun. 2020, 56, 2991.
doi: 10.1039/D0CC00702A pmid: 32610935 |
|
|
(g) Zhou, M.-B.; Li, Y.; Ouyang, X.; Li, J.-H. Sci. China: Chem. 2020, 63, 222.
doi: 10.1007/s11426-019-9633-x pmid: 32610935 |
|
| [4] |
For selected reviews, see: (a) Song, R.; Xie, Y. Chin. J. Chem. 2017, 35, 280.
doi: 10.1002/cjoc.v35.3 |
|
(b) Vessally, E.; Babazadeh, M.; Didehban, K.; Hosseinian, A.; Edjlali, L. Curr. Org. Chem. 2018, 22, 286.
doi: 10.2174/1385272821666170914111817 |
|
|
(c) Reddy, C. R.; Prajapti, S. K.; Warudikar, K.; Ranjan, R.; Rao, B. B. Org. Biomol. Chem. 2017, 15, 3130.
doi: 10.1039/C7OB00405B |
|
|
(d) Ni, S.; Zhou, J.; Mei, H.; Han, J. Tetrahedron Lett. 2018, 59, 1309.
|
|
| [5] |
Nair, A. M.; Shinde, A. H.; Kumar, S.; Volla, C. M. R. Chem. Commun. 2020, 56, 12367.
doi: 10.1039/D0CC04800C |
|
(b) Reddy, C. R.; Dattahari, H.; Subbarao, M.; Aila, M.; Prajapti, S. K. Org. Lett. 2020, 22, 5342.
doi: 10.1021/acs.orglett.0c01588 |
|
|
(c) Nair, A. M.; Halder, I.; Khan, S.; Volla, C. M. R. Adv. Synth. Catal. 2020, 362, 224.
doi: 10.1002/adsc.v362.1 |
|
|
(d) Liu, Y.; Wang, Q.-L.; Chen, Z.; Zhou, Q.; Xiong, B.-Q.; Zhang, P.-L.; Tang, K.-W. Chem. Commun. 2019, 55, 12212.
doi: 10.1039/C9CC05949K |
|
| [6] |
For selected recent examples, see: (a) Zhang, N.; Zuo, H.; Xu, C.; Pan, J.; Sun, J.; Guo, C. Chin. Chem. Lett. 2020, 31, 337.
doi: 10.1016/j.cclet.2019.06.008 |
|
(b) Chen, Y.; Chen, Y.-J.; Guan, Z.; He, Y.-Y. Tetrahedron 2019, 75, 130763.
doi: 10.1016/j.tet.2019.130763 |
|
|
(c) Wang, C.-S.; Roisnel, T. P.; Dixneuf, H.; Soule, J.-F. Adv. Synth. Catal. 2019, 361, 445.
doi: 10.1002/adsc.v361.3 |
|
| [7] |
Wang, Y.-C.; Liu, J.-B.; Zhou, H. W.; Xie, W.; Rojsittisak, P.; Qiu, G. J. Org. Chem. 2020, 85, 1906.
doi: 10.1021/acs.joc.9b02590 |
| [8] |
(a) Wang, Y.-C.; Wang, R.-X.; Qiu, G.; Zhou, H.; Xie, W.; Liu, J.-B. Org. Chem. Front. 2019, 6, 2471.
doi: 10.1039/C9QO00540D |
|
(b) Ren, S.-F.; Wang, Y.-C.; Liu, J.-B.; Qiu, G. Chin. J. Org. Chem. 2021, 41, 3652. (in Chinese)
doi: 10.6023/cjoc202104046 |
|
|
( 任尚峰, 王玉超, 刘晋彪, 邱观音生, 有机化学, 2021, 41, 3652.)
doi: 10.6023/cjoc202104046 |
|
|
(c) Yuan, S.; Zhou, H.; Gao, L.; Liu, J.-B.; Qiu, G. Org. Lett. 2018, 20, 562.
doi: 10.1021/acs.orglett.7b03671 |
|
|
(d) Qiu, G.; Li, Y.; Ma, L.; Zhou, H. Org. Chem. Front. 2017, 4, 1069.
doi: 10.1039/C6QO00840B |
|
|
(e) Lin, C.-K.; Zhang, C.-X.; Huang, K. K.; Wang, Y.-C.; Liu, J.-B. J. Jiangxi Univ. Sci. Technol. 2020, 41, 1. (in Chinese)
|
|
|
( 林川凯, 张彩霞, 黄棵棵, 王玉超, 刘晋彪, 江西理工大学学报, 2020, 41, 1.)
|
|
|
(f) Rodrigues, I.; Barcellos, A. M.; Belladonaet, A. L.; Roehrs, J. A.; Cargnelutti, R.; Alves, D.; Perin, G.; Schumacher, R. F. Tetrahedron 2018, 74, 4242.
doi: 10.1016/j.tet.2018.06.046 |
|
|
(g) Goulart, H. A.; Neto, J. S. S.; Barcellos, A. M.; Barcellos, T.; Silva, M. S.; Alves, D.; Jacob, R. G.; Lenardão, E. J.; Perin, G. Adv. Synth. Catal. 2019, 361, 3403.
doi: 10.1002/adsc.201900288 |
|
|
(h) Wang, Y.-C.; Huang, K.; Lai, X.; Shi, Z.; Liu, J.; Qiu, G. Org. Biomol. Chem. 2021, 19, 1940.
doi: 10.1039/D1OB00010A |
|
| [9] |
(a) Zaman, M.; Hasan, M.; Pehkow, A. A.; Van Hecke, K.; Van der Eycken, E. V. Pereshivko, O. P.; Peshkow, V. A. Adv. Synth. Catal. 2020, 362, 261.
doi: 10.1002/adsc.v362.1 |
|
(b) Singh, K.; Malviya, B. K.; Jaiswal, P. K.; Verma, V. P.; Chimni, S. S.; Sharma, S. Org. Lett. 2019, 21, 6726.
doi: 10.1021/acs.orglett.9b02340 |
|
| [10] |
Yugandhar, D.; Kuriakose, S.; Nanubolu, J. B.; Srivastava, A. K. Org. Lett. 2016, 18, 1040.
doi: 10.1021/acs.orglett.6b00164 pmid: 26902973 |
| [11] |
Reviews, see: (a) Ivaanova, A.; Arsenyan, P. Coord. Chem. Rev. 2018, 370, 55.
doi: 10.1016/j.ccr.2018.05.015 |
|
(b) Yu, L.; Wang, J.; Cao, H.; Ding, K.; Xu, Q. Chin. J. Org. Chem. 2014, 34, 1986. (in Chinese)
doi: 10.6023/cjoc201405004 |
|
|
( 俞磊, 王俊, 曹洪恩, 丁克鸿, 徐清, 有机化学, 2014, 34, 1986.)
doi: 10.6023/cjoc201405004 |
|
| [12] |
For selected recent example, see: (a) Belladona, A. L.; Cervo, R.; Alves, D.; Barcellos, T.; Cargnelutti, R.; Schumacher, R. F. Tetrahedron Lett. 2020, 61, 152035.
doi: 10.1016/j.tetlet.2020.152035 |
|
(b) Hua, G.; Cordes, D. B.; Slawin, A. M. Z.; Woollins, J. D. ACS Omerga 2020, 5, 11737.
|
|
| [13] |
For selected recent example, see: (a) Zhang, Q.-B.; Yuan, P.-F.; Kai, L.-L.; Liu, K.; Ban, Y.-L.; Wang, X.-Y.; Wu, L.-Z.; Liu, Q. Org. Lett. 2019, 21, 885.
doi: 10.1021/acs.orglett.8b03738 |
|
(b) Ding, C.; Yu, Y.; Yu, Q.; Xie, Z.; Zhou, Y.; Zhou, J.; Liang, G.; Song, Z. ChemCatChem 2018, 10, 5397.
doi: 10.1002/cctc.v10.23 |
|
|
(c) Liu, M.; Li, Y.; Yu, L.; Xu, Q.; Jiang, X. Sci. China: Chem. 2018, 61, 294.
doi: 10.1007/s11426-017-9158-y |
|
| [14] |
(a) Chen, J.-M.; Qi, L.; Zhang, L.; Li, L.-J.; Hou, C.-Y.; Li, W.; Wang, L.-J. J. Org. Chem. 2020, 85, 10924.
doi: 10.1021/acs.joc.0c01519 |
|
(b) Leng, T.; Wu, G.; Zhou, Y.; Gao, W.; Ding, J.; Huang, X.; Liu, M.; Wu, H. Adv. Synth. Catal. 2018, 360, 4336.
doi: 10.1002/adsc.v360.22 |
|
| [15] |
Perin, G.; Soares, L. K.; Hellwig, P. S.; Silva, M. S.; Neto, J. S. S.; Roehrs, J. A.; Barcellos, T.; Lenardao, E. J. New J. Chem. 2019, 43, 6323.
doi: 10.1039/C9NJ00526A |
| [16] |
Recchi, A. M. S.; Rosa, P. H. P.; Back, D. F.; Zeni, G. Org. Biomol. Chem. 2020, 18, 3544.
doi: 10.1039/d0ob00609b pmid: 32342088 |
| [17] |
(a) Hua, J.; Fang, Z.; Bian, M.; Ma, T.; Yang, M.; Xu, J.; Liu, C.; He, W.; Zhu, N.; Yang, Z.; Guo, K. ChemSusChem 2020, 13, 2053.
doi: 10.1002/cssc.v13.8 pmid: 31476121 |
|
(b) Sahoo, H.; Grandi, G. S.; Ramakrishna, I.; Baiya, M. Org. Biomol. Chem. 2019, 17, 10163.
doi: 10.1039/C9OB02177A pmid: 31476121 |
|
|
(c) Zhang, R.; Xu, P.; Wang, S.-Y.; Ji, S.-J. J. Org. Chem. 2019, 84, 12324.
doi: 10.1021/acs.joc.9b01626 pmid: 31476121 |
| [1] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [2] | 芦军, 李奇闯, 梁仁校, 贾义霞. 镍催化吡啶/喹啉鎓盐分子内去芳构化芳基加成反应[J]. 有机化学, 2023, 43(5): 1875-1882. |
| [3] | 庞明杨, 常宏宏, 冯璋, 张娟. 过渡金属催化吲哚的串联去芳构化反应研究进展[J]. 有机化学, 2023, 43(4): 1271-1291. |
| [4] | 刘鹏, 钟富明, 廖礼豪, 谭伟强, 赵晓丹. 炔烃参与的去芳构化反应构建螺环己二烯酮类化合物的研究进展[J]. 有机化学, 2023, 43(12): 4019-4035. |
| [5] | 张怀远, 许诺, 唐蓉萍, 石星丽. 手性高价碘试剂诱导的不对称去芳构化反应研究进展[J]. 有机化学, 2023, 43(11): 3784-3805. |
| [6] | 殷一樊, 李晨, 孙凯, 刘颖杰, 王薪. 烯烃自由基胺硒化: β-氨基硒醚的简易合成[J]. 有机化学, 2022, 42(5): 1431-1437. |
| [7] | 李珊, 曹原, 蒋绿齐. 烷基、芳基和氟烷基硒化反应的研究进展[J]. 有机化学, 2022, 42(2): 434-457. |
| [8] | 易荣楠, 刘冬娴, 吴啟林, 赵明明, 王勇, 王峥. 电化学氧化-碘促进丙酮α-H芳(烷)硒化制备α-芳(烷)硒基丙酮[J]. 有机化学, 2021, 41(9): 3726-3732. |
| [9] | 孙名扬, 徐坤, 郭兵兵, 曾程初. 空气氧化的铜催化苯甲酸衍生物邻位C(sp2)—H键的硒化反应[J]. 有机化学, 2021, 41(6): 2302-2309. |
| [10] | 王榕, 徐立晨, 卢逸, 姜波, 郝文娟. 利用三氟甲基磺酸钪催化的吲哚去芳构化反应合成3,3'-双吲哚衍生物[J]. 有机化学, 2021, 41(4): 1582-1590. |
| [11] | 许颖, 李晨, 孟建萍, 黄玉玲, 付纪源, 刘冰, 刘颖杰, 陈宁. 有机硒参与的硒环化反应研究进展[J]. 有机化学, 2021, 41(3): 1012-1030. |
| [12] | 闫强, 范荣, 刘斌斌, 苏帅松, 王勃, 姚团利, 谭嘉靖. 苯炔参与的去芳构化反应研究进展[J]. 有机化学, 2021, 41(2): 455-470. |
| [13] | 王薪, 张艳, 孙凯, 孟建萍, 张冰. 光电技术在含硒杂环合成中的应用研究[J]. 有机化学, 2021, 41(12): 4588-4609. |
| [14] | 何树华, 张行, 吴红谕, 周诗雨, 肖垚, 游贤会, 陈锦杨. ICl催化氨基香豆素衍生物Csp2—H芳(烷)硒化反应研究[J]. 有机化学, 2021, 41(11): 4378-4383. |
| [15] | 芦军, 梁仁校, 贾义霞. 铜催化萘胺分子内去芳构化芳基化反应[J]. 有机化学, 2021, 41(10): 4007-4013. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||