有机化学 ›› 2022, Vol. 42 ›› Issue (1): 147-159.DOI: 10.6023/cjoc202108006 上一篇 下一篇
所属专题: 有机氟化学虚拟合辑
研究论文
收稿日期:2021-08-06
修回日期:2021-09-05
发布日期:2021-09-14
通讯作者:
陆熹, 傅尧
基金资助:
Zhe Chang, Jiaxin Wang, Xi Lu( ), Yao Fu(
), Yao Fu( )
)
Received:2021-08-06
Revised:2021-09-05
Published:2021-09-14
Contact:
Xi Lu, Yao Fu
Supported by:文章分享
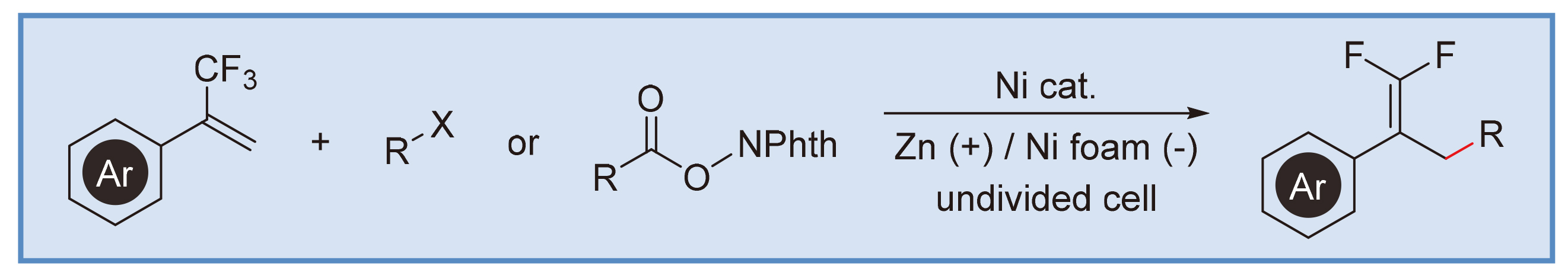
偕二氟烯烃是一类重要的含氟有机化合物, 在有机合成化学和药物化学研究领域展现出独特的结构优势. 例如, 偕二氟烯基可以便利地转化为单氟烯基、二氟烷基、三氟甲基以及其他多种含氟结构. 偕二氟烯基结构作为羰基理想的电子等排体在药物设计研究中也有广泛的应用. 报道了一种镍促进的电化学还原交叉偶联反应合成功能化的偕二氟烯烃. 该反应在非分隔电解槽中进行, 在温和的电化学还原条件下, 实现了三氟甲基烯烃烯丙基脱氟、氧化还原活性羧酸酯脱羧或者烷基卤化物脱卤素的有机结合. 反应可有效避免使用化学计量的金属粉末或有机还原剂. 该反应为含偕二氟烯基功能结构的生物活性分子提供了有效的合成途径.
常哲, 王佳鑫, 陆熹, 傅尧. 镍促进电化学还原交叉偶联合成偕二氟烯烃[J]. 有机化学, 2022, 42(1): 147-159.
Zhe Chang, Jiaxin Wang, Xi Lu, Yao Fu. Synthesis of gem-Difluoroalkenes through Nickel-Promoted Electrochemical Reductive Cross-Coupling[J]. Chinese Journal of Organic Chemistry, 2022, 42(1): 147-159.
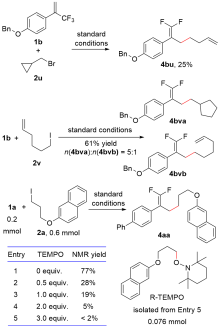
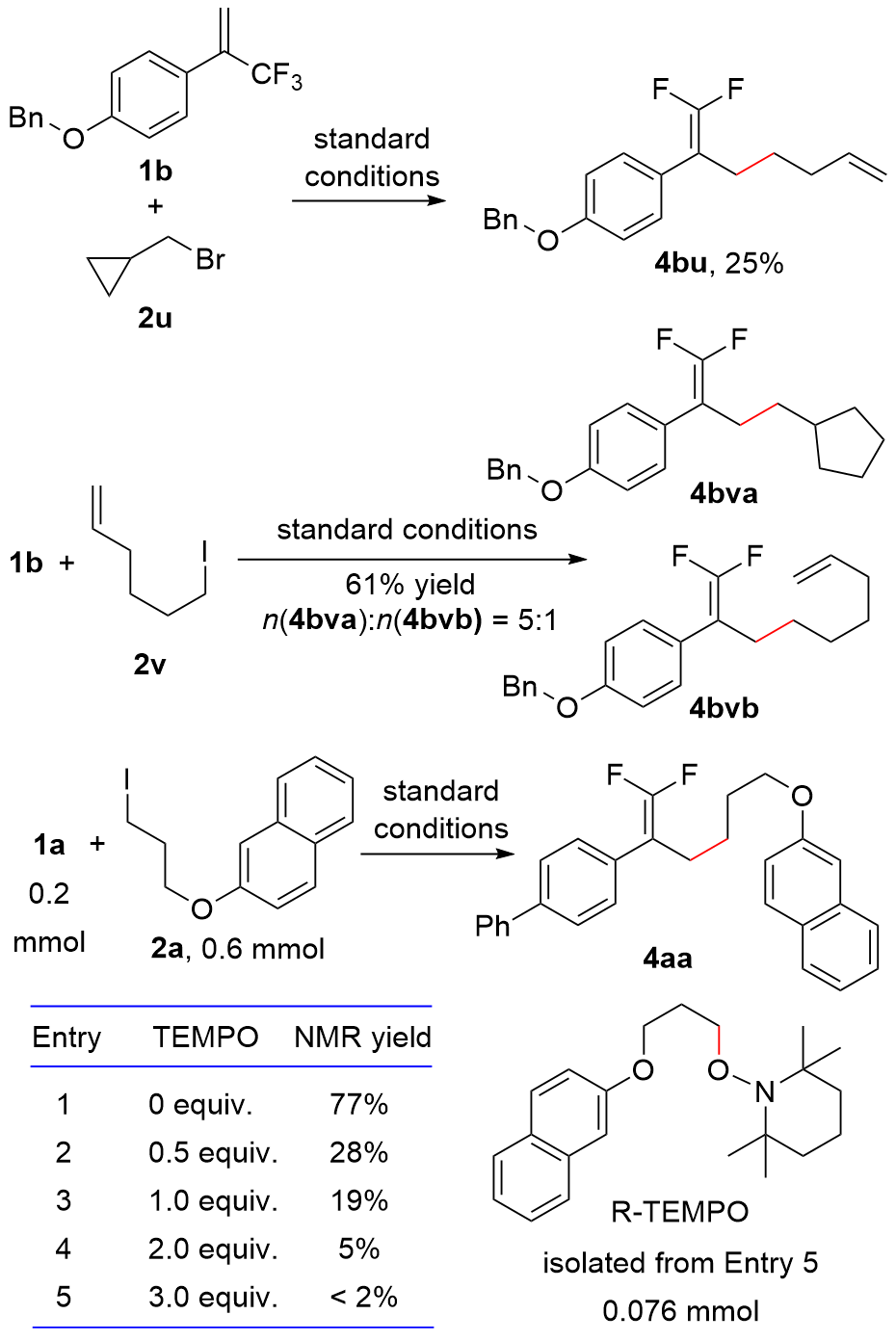
| Entry | Variation from standard conditions | Conversionb/% of 1a | Yieldb/% of 4aa | |
|---|---|---|---|---|
| 1 | No | 81 | 77 (73c) | |
| 2 | NiCl2 in lieu of NiBr2(diglyme) | 86 | 76 | |
| 3 | Ni(acac)2 in lieu of NiBr2(diglyme) | 76 | 52 | |
| 4 | L1 in lieu of Pybox | 74 | 40 | |
| 5 | L2 in lieu of Pybox | 62 | 41 | |
| 6 | L3 in lieu of Pybox | 52 | 48 | |
| 7 | KBr in lieu of nBu4NBr | 78 | 72 | |
| 8 | LiBr in lieu of nBu4NBr | 77 | 62 | |
| 9 | 0.5 equiv. nBu4NBr | 76 | 75 | |
| 10 | 2.0 equiv. nBu4NBr | 74 | 70 | |
| 11 | DMAc in lieu of DMSO | 66 | 57 | |
| 12 | DMF in lieu of DMSO | 78 | 53 | |
| 13 | CH3CN in lieu of DMSO | 88 | <2 | |
| 14 | n(1a)∶n(2a)=1∶2 | 84 | 70 | |
| 15 | n(1a)∶n(2a)=2∶1 | 66 | 53 | |
| 16 | No NiBr2(diglyme) | 79 | 44 | |
| 17 | No electric current | 41 | 39 | |
| 18 | No NiBr2(diglyme), no electric current | 25 | 15 | |
| 19 | Fe(+) in lieu of Zn(+) | 70 | 55 | |
| 20 | Al(+) in lieu of Zn(+) | 95 | <2 | |
| 21 | Fe powder in lieu of electric current | <2 | <2 | |
| 22 | Al powder in lieu of electric current | <2 | <2 | |
| 23 | RVC(—) in lieu of Ni foam(—) | 87 | 73 | |
| 24 | RVC(—) in lieu of Ni foam(—), no NiBr2(diglyme) | 82 | 10 | |
| 25 | Graphite(—) in lieu of Ni foam(—) | 69 | 48 | |
| 26 | Graphite(—) in lieu of Ni foam(—), no NiBr2(diglyme) | 60 | 13 | |
| 27 | Pt(—) in lieu of Ni foam(—) | 81 | 35 | |
| 28 | 3a in lieu of 2a | 89 | 88 (84c) | |
| 29 | 3a in lieu of 2a, no NiBr2(diglyme) | 83 | 64 | |
| 30 | 3a in lieu of 2a, no electric current | 70 | 69 | |
| 31 | 3a in lieu of 2a, no NiBr2(diglyme), no electric current | 69 | 67 | |
| 32 | 3a in lieu of 2a, Fe(+) in lieu of Zn(+) | 60 | 42 | |
| 33 | 3a in lieu of 2a, Al(+) in lieu of Zn(+) | >99 | 31 | |
| 34 | 3a in lieu of 2a, Fe powder in lieu of electric current | <2 | <2 | |
| 35 | 3a in lieu of 2a, Al powder in lieu of electric current | <2 | <2 | |
| 36 | 3a in lieu of 2a, n(1a)∶n(3a)=1∶1.5 | 50 | 50 | |
| 37 | 3a in lieu of 2a, n(1a)∶n(3a)=1∶2 | 77 | 52 | |
| 38 | 3a in lieu of 2a, n(1a)∶n(3a)=1∶2.5 | 82 | 76 | |
| 39 | 3a in lieu of 2a, n(1a)∶n(3a)=2.5∶1 | 70 | 37 | |
| Entry | Variation from standard conditions | Conversionb/% of 1a | Yieldb/% of 4aa | |
|---|---|---|---|---|
| 1 | No | 81 | 77 (73c) | |
| 2 | NiCl2 in lieu of NiBr2(diglyme) | 86 | 76 | |
| 3 | Ni(acac)2 in lieu of NiBr2(diglyme) | 76 | 52 | |
| 4 | L1 in lieu of Pybox | 74 | 40 | |
| 5 | L2 in lieu of Pybox | 62 | 41 | |
| 6 | L3 in lieu of Pybox | 52 | 48 | |
| 7 | KBr in lieu of nBu4NBr | 78 | 72 | |
| 8 | LiBr in lieu of nBu4NBr | 77 | 62 | |
| 9 | 0.5 equiv. nBu4NBr | 76 | 75 | |
| 10 | 2.0 equiv. nBu4NBr | 74 | 70 | |
| 11 | DMAc in lieu of DMSO | 66 | 57 | |
| 12 | DMF in lieu of DMSO | 78 | 53 | |
| 13 | CH3CN in lieu of DMSO | 88 | <2 | |
| 14 | n(1a)∶n(2a)=1∶2 | 84 | 70 | |
| 15 | n(1a)∶n(2a)=2∶1 | 66 | 53 | |
| 16 | No NiBr2(diglyme) | 79 | 44 | |
| 17 | No electric current | 41 | 39 | |
| 18 | No NiBr2(diglyme), no electric current | 25 | 15 | |
| 19 | Fe(+) in lieu of Zn(+) | 70 | 55 | |
| 20 | Al(+) in lieu of Zn(+) | 95 | <2 | |
| 21 | Fe powder in lieu of electric current | <2 | <2 | |
| 22 | Al powder in lieu of electric current | <2 | <2 | |
| 23 | RVC(—) in lieu of Ni foam(—) | 87 | 73 | |
| 24 | RVC(—) in lieu of Ni foam(—), no NiBr2(diglyme) | 82 | 10 | |
| 25 | Graphite(—) in lieu of Ni foam(—) | 69 | 48 | |
| 26 | Graphite(—) in lieu of Ni foam(—), no NiBr2(diglyme) | 60 | 13 | |
| 27 | Pt(—) in lieu of Ni foam(—) | 81 | 35 | |
| 28 | 3a in lieu of 2a | 89 | 88 (84c) | |
| 29 | 3a in lieu of 2a, no NiBr2(diglyme) | 83 | 64 | |
| 30 | 3a in lieu of 2a, no electric current | 70 | 69 | |
| 31 | 3a in lieu of 2a, no NiBr2(diglyme), no electric current | 69 | 67 | |
| 32 | 3a in lieu of 2a, Fe(+) in lieu of Zn(+) | 60 | 42 | |
| 33 | 3a in lieu of 2a, Al(+) in lieu of Zn(+) | >99 | 31 | |
| 34 | 3a in lieu of 2a, Fe powder in lieu of electric current | <2 | <2 | |
| 35 | 3a in lieu of 2a, Al powder in lieu of electric current | <2 | <2 | |
| 36 | 3a in lieu of 2a, n(1a)∶n(3a)=1∶1.5 | 50 | 50 | |
| 37 | 3a in lieu of 2a, n(1a)∶n(3a)=1∶2 | 77 | 52 | |
| 38 | 3a in lieu of 2a, n(1a)∶n(3a)=1∶2.5 | 82 | 76 | |
| 39 | 3a in lieu of 2a, n(1a)∶n(3a)=2.5∶1 | 70 | 37 | |
| [1] |
(a) Zhang, X.; Cao, S. Tetrahedron Lett. 2017, 58, 375.
doi: 10.1016/j.tetlet.2016.12.054 |
|
(b) Du, B.; Chan, C.-M.; Lee, P.-Y.; Cheung, L.-H.; Xu, X.; Lin, Z.; Yu, W.-Y. Nat. Commun. 2021, 12, 412.
doi: 10.1038/s41467-020-20725-9 |
|
|
(c) Zhang, X.-J.; Cheng, Y.-M.; Zhao, X.-W.; Cao, Z.-Y.; Xiao, X.; Xu, Y. Org. Chem. Front. 2021, 8, 2315.
doi: 10.1039/D0QO01630F |
|
|
(d) Zhuo, K.-F.; Xu, W.-Y.; Gong, T.-J.; Fu, Y. Chem. Commun. 2020, 56, 2340.
doi: 10.1039/C9CC08485A |
|
|
(e) Ma, X.; Song, Q. Chem. Soc. Rev. 2020, 49, 9197.
doi: 10.1039/D0CS00604A |
|
|
(f) Sheng, J.; Wu, N.; Liu, X.; Liu, F.; Liu, S.; Ding, W.; Liu, C.; Cheng, X. Chin. J. Org. Chem. 2020, 40, 3873. (in Chinese)
doi: 10.6023/cjoc202006071 |
|
|
(盛杰, 吴娜, 刘旭, 刘峰, 刘帅, 丁伟杰, 刘畅, 程旭, 有机化学, 2020, 40, 3873.)
doi: 10.6023/cjoc202006071 |
|
|
(g) Shen, X.; Liu, Q.; Ni, C.; Hu, J. Chin. J. Chem. 2014, 32, 703.
doi: 10.1002/cjoc.201400403 |
|
|
(h) Xiong, B.; Wang, T.; Sun, H.; Li, Y.; Kramer, S.; Cheng, G.-J.; Lian, Z. ACS Catal. 2020, 10, 13616.
doi: 10.1021/acscatal.0c03993 |
|
| [2] |
(a) Fujita, T.; Fuchibe, K.; Ichikawa, J. Angew. Chem., Int. Ed. 2018, 58, 390.
doi: 10.1002/anie.v58.2 |
|
(b) Cai, Y.; Tan, D.; Zhang, Q.; Lü, W.; Li, Q.; Wang, H. Chin. Chem. Lett. 2021, 32, 417.
doi: 10.1016/j.cclet.2020.03.031 |
|
| [3] |
(a) He, S.-J.; Pi, J.-J.; Li, Y.; Lu, X.; Fu, Y. Acta Chim. Sinica 2018, 76, 956. (in Chinese)
doi: 10.6023/A18080333 |
|
(何世江, 皮静静, 李炎, 陆熹, 傅尧, 化学学报, 2018, 76, 956.)
doi: 10.6023/A18080333 |
|
|
(b) Song, S.; Liu, H.; Wang, L.; Zhu, C.; Loh, T. P.; Feng, C. Chin. J. Chem. 2019, 37, 1036.
doi: 10.1002/cjoc.v37.10 |
|
|
(c) Gao, P.; Wang, G.; Xi, L.; Wang, M.; Li, S.; Shi, Z. Chin. J. Chem. 2019, 37, 1009.
doi: 10.1002/cjoc.v37.10 |
|
|
(d) Zhang, J.; Wang, B.; Liu, Y.; Cao, S. Chin. J. Org. Chem. 2019, 39, 249. (in Chinese)
doi: 10.6023/cjoc201807013 |
|
|
(张娟, 王碧云, 刘熠森, 曹松, 有机化学, 2019, 39, 249.)
doi: 10.6023/cjoc201807013 |
|
|
(e) He, S.-Y.; Zhang, X.-G. Org. Chem. Front. 2020, 7, 3174.
doi: 10.1039/D0QO00818D |
|
| [4] |
(a) Hu, X.-S.; Ding, P.-G.; Yu, J.-S.; Zhou, J. Org. Chem. Front. 2019, 6, 2500.
doi: 10.1039/C9QO00577C |
|
(b) Huang, X.; Zhao, W.; Liang, Y.; Wang, M.; Zhan, Y.; Zhang, Y.; Kong, L.; Wang, Z.-X.; Peng, B. Org. Chem. Front. 2021, 8, 1280.
doi: 10.1039/D0QO01513J |
|
| [5] |
(a) Lin, T.-Y.; Pan, Z.; Tu, Y.; Zhu, S.; Wu, H.-H.; Liu, Y.; Li, Z.; Zhang, J. Angew. Chem., Int. Ed. 2020, 59, 22957.
doi: 10.1002/anie.v59.51 |
|
(b) Hu, J.; Yang, Y.; Lou, Z.; Ni, C.; Hu, J. Chin. J. Chem. 2018, 36, 1202.
doi: 10.1002/cjoc.v36.12 |
|
| [6] |
Magueur, G.; Crousse, B.; Ourévitch, M.; Bonnet-Delpon, D.; Bégué, J.-P. J. Fluorine Chem. 2006, 127, 637.
doi: 10.1016/j.jfluchem.2005.12.013 |
| [7] |
(a) Qin, W.; Chen, J.; Xiong, W.; Liu, G. Chin. J. Org. Chem. 2020, 40, 3177. (in Chinese)
doi: 10.6023/cjoc202005016 |
|
(秦文兵, 陈嘉怡, 熊威, 刘国凯, 有机化学, 2020, 40, 3177.)
doi: 10.6023/cjoc202005016 |
|
|
(b) Jin, Y.; Wang, Y.; Bao, K.; Sheng, R.; Tao, X. Chin. J. Org. Chem. 2019, 39, 2726. (in Chinese)
doi: 10.6023/cjoc201903063 |
|
|
(陶雪芬, 盛荣, 鲍堃, 王玉新, 金银秀, 有机化学, 2019, 39, 2726.)
doi: 10.6023/cjoc201903063 |
|
|
(c) Xie, Q.; Hu, J. Chin. J. Chem. 2020, 38, 202.
doi: 10.1002/cjoc.v38.2 |
|
| [8] |
(a) Zhao, Y.; Huang, W.; Zhu, L.; Hu, J. Org. Lett. 2010, 12, 1444.
doi: 10.1021/ol100090r |
|
(b) Hu, M.; He, Z.; Gao, B.; Li, L.; Ni, C.; Hu, J. J. Am. Chem. Soc. 2013, 135, 17302.
doi: 10.1021/ja409941r |
|
|
(c) Hu, M.; Ni, C.; Li, L.; Han, Y.; Hu, J. J. Am. Chem. Soc. 2015, 137, 14496.
doi: 10.1021/jacs.5b09888 |
|
| [9] |
(a) Zheng, J.; Cai, J.; Lin, J.-H.; Guo, Y.; Xiao, J.-C. Chem. Commun. 2013, 49, 7513.
doi: 10.1039/c3cc44271c |
|
(b) Yu, J.; Lin, J.; Xiao, J. Chin. J. Org. Chem. 2019, 39, 265. (in Chinese)
|
|
|
(于蛟, 林锦鸿, 肖吉昌, 有机化学, 2019, 39, 265.)
doi: 10.6023/cjoc201806024 |
|
| [10] |
Zhang, Z.; Yu, W.; Wu, C.; Wang, C.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2016, 55, 273.
doi: 10.1002/anie.201509711 |
| [11] |
(a) Zeng, H.; Cai, Y.; Jiang, H.; Zhu, C. Org. Lett. 2021, 23, 66.
doi: 10.1021/acs.orglett.0c03708 pmid: 30680998 |
|
(b) Liu, Y.; Zhou, Y.; Zhao, Y.; Qu, J. Org. Lett. 2017, 19, 946.
doi: 10.1021/acs.orglett.7b00168 pmid: 30680998 |
|
|
(c) Zeng, H.; Zhu, C.; Jiang, H. Org. Lett. 2019, 21, 1130.
doi: 10.1021/acs.orglett.9b00074 pmid: 30680998 |
|
|
(d) Cai, Y.; Zeng, H.; Zhu, C.; Liu, C.; Liu, G.; Jiang, H. Org. Chem. Front. 2020, 7, 1260.
doi: 10.1039/D0QO00121J pmid: 30680998 |
|
|
(e) Wu, X.; Xie, F.; Gridnev, I. D.; Zhang, W. Org. Lett. 2018, 20, 1638.
doi: 10.1021/acs.orglett.8b00379 pmid: 30680998 |
|
|
(f) Gao, P.; Yuan, C.; Zhao, Y.; Shi, Z. Chem 2018, 4, 2201.
doi: 10.1016/j.chempr.2018.07.003 pmid: 30680998 |
|
|
(g) Gao, P.; Gao, L.; Xi, L.; Zhang, Z.; Li, S.; Shi, Z. Org. Chem. Front. 2020, 7, 2618.
doi: 10.1039/D0QO00773K pmid: 30680998 |
|
|
(h) Chen, G.; Wang, L.; Liu, X.; Liu, P. Adv. Synth. Catal. 2020, 362, 2990.
doi: 10.1002/adsc.v362.14 pmid: 30680998 |
|
|
(i) Yao, C.; Wang, S.; Norton, J.; Hammond, M. J. Am. Chem. Soc. 2020, 142, 4793.
doi: 10.1021/jacs.9b13757 pmid: 30680998 |
|
|
(j) Chen, F.; Xu, X.; He, Y.; Huang, G.; Zhu, S. Angew. Chem., Int. Ed. 2020, 59, 5398.
doi: 10.1002/anie.v59.13 pmid: 30680998 |
|
|
(k) Fuchibe, K.; Hatta, H.; Oh, K.; Oki, R.; Ichikawa, J. Angew. Chem., Int. Ed. 2017, 56, 5890.
doi: 10.1002/anie.201701985 pmid: 30680998 |
|
|
(l) Fuchibe, K.; Takahashi, M.; Ichikawa, J. Angew. Chem., Int. Ed. 2012, 51, 12059.
doi: 10.1002/anie.201206946 pmid: 30680998 |
|
|
(m) Ichitsuka, T.; Fujita, T.; Arita, T.; Ichikawa, J. Angew. Chem., Int. Ed. 2014, 53, 7564.
doi: 10.1002/anie.201402695 pmid: 30680998 |
|
|
(n) Ichitsuka, T.; Fujita, T.; Ichikawa, J. ACS Catal. 2015, 5, 5947.
doi: 10.1021/acscatal.5b01463 pmid: 30680998 |
|
|
(o) Jaroschik, F. Chem. Eur. J. 2018, 24, 14572.
doi: 10.1002/chem.v24.55 pmid: 30680998 |
|
|
(p) Kojima, R.; Akiyama, S.; Ito, H. Angew. Chem., Int. Ed. 2018, 57, 7196.
doi: 10.1002/anie.v57.24 pmid: 30680998 |
|
|
(q) Kojima, R.; Kubota, K.; Ito, H. Chem. Commun. 2017, 53, 10688.
doi: 10.1039/C7CC05225A pmid: 30680998 |
|
|
(r) Kojima, Y.; Takata, T.; Hirano, K.; Miura, M. Chem. Lett. 2020, 49, 637.
doi: 10.1246/cl.200163 pmid: 30680998 |
|
|
(s) Liu, Y.; Li, C.; Liu, C.; He, J.; Zhao, X.; Cao, S. Tetrahedron Lett. 2020, 61, 151940.
doi: 10.1016/j.tetlet.2020.151940 pmid: 30680998 |
|
|
(t) Liu, Z.; Tu, X.-S.; Guo, L.-T.; Wang, X.-C. Chem. Sci. 2020, 11, 11548.
doi: 10.1039/D0SC03883K pmid: 30680998 |
|
|
(u) Yan, S.-S.; Wu, D.-S.; Ye, J.-H.; Gong, L.; Zeng, X.; Ran, C.-K.; Gui, Y.-Y.; Li, J.; Yu, D.-G. ACS Catal. 2019, 9, 6987.
doi: 10.1021/acscatal.9b02351 pmid: 30680998 |
|
|
(v) Pan, Q.; Ping, Y.; Wang, Y.; Guo, Y.; Kong, W. J. Am. Chem. Soc. 2021, 143, 10282.
doi: 10.1021/jacs.1c03827 pmid: 30680998 |
|
|
(w) Tian, F.; Yan, G.; Yu, J. Chem. Commun. 2019, 55, 13486.
doi: 10.1039/C9CC06465F pmid: 30680998 |
|
|
(x) Xie, S.-L.; Cui, X.-Y.; Gao, X.-T.; Zhou, F.; Wu, H.-H.; Zhou, J. Org. Chem. Front. 2019, 6, 3678.
doi: 10.1039/C9QO00923J pmid: 30680998 |
|
|
(y) Li, Y.; Wang, Y.; Zhu, L.; Qu, L.; Lan, Y. Chin. J. Org. Chem. 2019, 39, 38. (in Chinese)
doi: 10.6023/cjoc201810020 pmid: 30680998 |
|
|
(李园园, 王元鉴, 朱磊, 屈凌波, 蓝宇, 有机化学, 2019, 39, 38.)
doi: 10.6023/cjoc201810020 pmid: 30680998 |
|
| [12] |
Dai, W.; Lin, Y.; Wan, Y.; Cao, S. Org. Chem. Front. 2018, 5, 55.
doi: 10.1039/C7QO00716G |
| [13] |
Wang, M.; Pu, X.; Zhao, Y.; Wang, P.; Li, Z.; Zhu, C.; Shi, Z. J. Am. Chem. Soc. 2018, 140, 9061.
doi: 10.1021/jacs.8b04902 |
| [14] |
Miura, T.; Ito, Y.; Murakami, M. Chem. Lett. 2008, 37, 1006.
doi: 10.1246/cl.2008.1006 |
| [15] |
Huang, Y.; Hayashi, T. J. Am. Chem. Soc. 2016, 138, 12340.
doi: 10.1021/jacs.6b07844 |
| [16] |
Cheng, R.; Xu, C.; Zhang, X. Chin. J. Org. Chem. 2020, 40, 3307. (in Chinese)
doi: 10.6023/cjoc202005082 |
|
(程然, 徐畅, 张新刚, 有机化学, 2020, 40, 3307.)
doi: 10.6023/cjoc202005082 |
|
| [17] |
(a) Phelan, J. P.; Wiles, R. J.; Lang, S. B.; Kelly, C. B.; Molander, G. A. Chem. Sci. 2018, 9, 3215.
doi: 10.1039/c7sc05420c pmid: 29732105 |
|
(b) Lang, S. B.; Wiles, R. J.; Kelly, C. B.; Molander, G. A. Angew. Chem., Int. Ed. 2017, 56, 15073.
doi: 10.1002/anie.201709487 pmid: 29732105 |
|
|
(c) Wiles, R. J.; Phelan, J. P.; Molander, G. A. Chem. Commun. 2019, 55, 7599.
doi: 10.1039/C9CC04265B pmid: 29732105 |
|
| [18] |
(a) Chen, H.; Anand, D.; Zhou, L. Asian J. Org. Chem. 2019, 8, 661.
doi: 10.1002/ajoc.v8.5 |
|
(b) Li, L.; Xiao, T.; Chen, H.; Zhou, L. Chem. Eur. J. 2017, 23, 2249.
doi: 10.1002/chem.201605919 |
|
|
(c) Anand, D.; Sun, Z.; Zhou, L. Org. Lett. 2020, 22, 2371.
doi: 10.1021/acs.orglett.0c00568 |
|
|
(d) Chen, H.; Xiao, T.; Li, L.; Anand, D.; He, Y.; Zhou, L. Adv. Synth. Catal. 2017, 359, 3642.
doi: 10.1002/adsc.v359.20 |
|
|
(e) He, Y.; Anand, D.; Sun, Z.; Zhou, L. Org. Lett. 2019, 21, 3769.
doi: 10.1021/acs.orglett.9b01210 |
|
|
(f) Xiao, T.; Li, L.; Zhou, L. J. Org. Chem. 2016, 81, 7908.
doi: 10.1021/acs.joc.6b01620 |
|
| [19] |
(a) Guo, Y.-Q.; Wang, R.; Song, H.; Liu, Y.; Wang, Q. Org. Lett. 2020, 22, 709.
doi: 10.1021/acs.orglett.9b04504 |
|
(b) Guo, Y.-Q.; Wu, Y.; Wang, R.; Song, H.; Liu, Y.; Wang, Q. Org. Lett. 2021, 23, 2353.
doi: 10.1021/acs.orglett.1c00546 |
|
|
(c) Xiang, P.; He, L.; Li, H.; Qi, Z.; Zhang, M.; Fu, Q.; Wei, J.; Du, X.; Yi, D.; Wei, S. Tetrahedron Lett. 2020, 61, 152369.
doi: 10.1016/j.tetlet.2020.152369 |
|
| [20] |
Lu, X.; Wang, Y.; Zhang, B.; Pi, J.-J.; Wang, X.-X.; Gong, T.-J.; Xiao, B.; Fu, Y. J. Am. Chem. Soc. 2017, 139, 12632.
doi: 10.1021/jacs.7b06469 pmid: 28849923 |
| [21] |
Lu, X.; Wang, X.-X.; Gong, T.-J.; Pi, J.-J.; He, S.-J.; Fu, Y. Chem. Sci. 2019, 10, 809.
doi: 10.1039/C8SC04335C |
| [22] |
Lan, Y.; Yang, F.; Wang, C. ACS Catal. 2018, 8, 9245.
doi: 10.1021/acscatal.8b02784 |
| [23] |
(a) Yu, L.; Tang, M.-L.; Si, C.-M.; Meng, Z.; Liang, Y.; Han, J.; Sun, X. Org. Lett. 2018, 20, 4579.
doi: 10.1021/acs.orglett.8b01866 |
|
(b) Du, H.-W.; Chen, Y.; Sun, J.; Gao, Q.-S.; Wang, H.; Zhou, M.-D. S. Org. Lett. 2020, 22, 9342.
doi: 10.1021/acs.orglett.0c03554 |
|
|
(c) Li, Z.; Wang, K.-F.; Zhao, X.; Ti, H.; Liu, X.-G.; Wang, H. Nat. Commun. 2020, 11, 5036.
doi: 10.1038/s41467-020-18834-6 |
|
|
(d) Lin, Z.; Lan, Y.; Wang, C. Org. Lett. 2019, 21, 8316.
doi: 10.1021/acs.orglett.9b03102 |
|
| [24] |
(a) Ding, D.; Lan, Y.; Lin, Z.; Wang, C. Org. Lett. 2019, 21, 2723.
doi: 10.1021/acs.orglett.9b00692 |
|
(b) Lin, Z.; Lan, Y.; Wang, C. Org. Lett. 2020, 22, 3509.
doi: 10.1021/acs.orglett.0c00960 |
|
|
(c) Lu, X.-Y.; Jiang, R.-C.; Li, J.-M.; Liu, C.-C.; Wang, Q.-Q.; Zhou, H.-P. Org. Biomol. Chem. 2020, 18, 3674.
doi: 10.1039/D0OB00535E |
|
|
(d) Fan, P.; Zhang, C.; Lan, Y.; Lin, Z.; Zhang, L.; Wang, C. Chem. Commun. 2019, 55, 12691.
doi: 10.1039/C9CC07285C |
|
|
(e) Jin, Y.; Wu, J.; Lin, Z.; Lan, Y.; Wang, C. Org. Lett. 2020, 22, 5347.
doi: 10.1021/acs.orglett.0c01592 |
|
|
(f) Zhu, C.; Liu, Z.-Y.; Tang, L.; Zhang, H.; Zhang, Y.-F.; Walsh, P. J.; Feng, C. Nat. Commun. 2020, 11, 4860.
doi: 10.1038/s41467-020-18658-4 |
|
| [25] |
(a) Kawamata, Y.; Vantourout, J. C.; Hickey, D. P.; Bai, P.; Chen, L.; Hou, Q.; Qiao, W.; Barman, K.; Edwards, M. A.; Garrido-Castro, A. F.; deGruyter, J. N.; Nakamura, H.; Knouse, K.; Qin, C.; Clay, K. J.; Bao, D.; Li, C.; Starr, J. T.; Garcia-Irizarry, C.; Sach, N.; White, H. S.; Neurock, M.; Minteer, S. D.; Baran, P. S. J. Am. Chem. Soc. 2019, 141, 6392.
doi: 10.1021/jacs.9b01886 pmid: 30905151 |
|
(b) Kim, H.; Kim, H.; Lambert, T. H.; Lin, S. J. Am. Chem. Soc. 2020, 142, 2087.
doi: 10.1021/jacs.9b10678 pmid: 30905151 |
|
|
(c) Li, C.; Kawamata, Y.; Nakamura, H.; Vantourout, J. C.; Liu, Z.; Hou, Q.; Bao, D.; Starr, J. T.; Chen, J.; Yan, M.; Baran, P. S. Angew. Chem., Int. Ed. 2017, 56, 13088.
doi: 10.1002/anie.201707906 pmid: 30905151 |
|
|
(d) Wang, P.; Yang, Z.; Wang, Z.; Xu, C.; Huang, L.; Wang, S.; Zhang, H.; Lei, A. Angew. Chem., Int. Ed. 2019, 58, 15747.
doi: 10.1002/anie.v58.44 pmid: 30905151 |
|
|
(e) Liu, Q.; Sun, B.; Liu, Z.; Kao, Y.; Dong, B.-W.; Jiang, S.-D.; Li, F.; Liu, G.; Yang, Y.; Mo, F. Chem. Sci. 2018, 9, 8731.
doi: 10.1039/C8SC03346C pmid: 30905151 |
|
|
(f) Liu, D.; Ma, H.-X.; Fang, P.; Mei, T.-S. Angew. Chem., Int. Ed. 2019, 58, 5033.
doi: 10.1002/anie.v58.15 pmid: 30905151 |
|
|
(g) Zou, Z.; Zhang, W.; Wang, Y.; Pan, Y. Org. Chem. Front. 2021, 8, 2786.
doi: 10.1039/D1QO00054C pmid: 30905151 |
|
|
(h) Lu, L.; Li, H.; Zheng, Y.; Bu, F.; Lei, A. CCS Chem. 2020, 2, 2669.
pmid: 30905151 |
|
|
(i) Dou, G.-Y.; Jiang, Y.-Y.; Xu, K.; Zeng, C.-C. Org. Chem. Front. 2019, 6, 2392.
doi: 10.1039/C9QO00552H pmid: 30905151 |
|
|
(j) Liang, K.; Wang, S.; Cong, H.; Lu, L.; Lei, A. CCS Chem. 2021, 3, 1727.
pmid: 30905151 |
|
|
(k) Huang, C.; Xu, H.-C. Sci. China Chem. 2019, 62, 1501.
doi: 10.1007/s11426-019-9554-1 pmid: 30905151 |
|
|
(l) Feng, E.; Hou, Z.; Xu, H. Chin. J. Org. Chem. 2019, 39, 1424. (in Chinese)
doi: 10.6023/cjoc201812007 pmid: 30905151 |
|
|
(冯恩祺, 侯中伟, 徐海超, 有机化学, 2019, 39, 1424.)
doi: 10.6023/cjoc201812007 pmid: 30905151 |
|
|
(m) Zhang, C.; Bu, F.; Zeng, C.; Wang, D.; Lu, L.; Zhang, H.; Lei, A. CCS Chem. 2021, 3, 1404.
pmid: 30905151 |
|
|
(n) Deng, Y.; Lu, F.; You, S.; Xia, T.; Zheng, Y.; Lu, C.; Yang, G.; Chen, Z.; Gao, M.; Lei, A. Chin. J. Chem. 2019, 37, 817.
doi: 10.1002/cjoc.v37.8 pmid: 30905151 |
|
|
(o) Hou, Z. W.; Xu, H. C. Chin. J. Chem. 2020, 38, 394.
doi: 10.1002/cjoc.v38.4 pmid: 30905151 |
|
|
(p) Liu, S.; Zhao, W.; Li, J.; Wu, N.; Liu, C.; Wang, X.; Li, S.; Zhu, Y.; Liang, Y.; Cheng, X. CCS Chem. 2021, 3, 872.
pmid: 30905151 |
|
|
(q) Qiu, Y.; Zhu, C.; Stangier, M.; Struwe, J.; Ackermann, L. CCS Chem. 2020, 2, 1529.
pmid: 30905151 |
|
|
(r) Xu, Y.; Lin, L.; Han, Y.; Liu, Y. Chin. J. Org. Chem. 2021, 41, 934. (in Chinese)
doi: 10.6023/cjoc202008017 pmid: 30905151 |
|
|
(许颖, 林立青, 韩莹徽, 刘颖杰, 有机化学, 2021, 41, 934.)
doi: 10.6023/cjoc202008017 pmid: 30905151 |
|
|
(s) Yang, Q.-L.; Wang, X.-Y.; Weng, X.-J.; Yang, X.; Xu, X.-T.; Tong, X.; Fang, P.; Wu, X.-Y.; Mei, T.-S. Acta Chim. Sinica 2019, 77, 866. (in Chinese)
doi: 10.6023/A19040135 pmid: 30905151 |
|
|
(杨启亮, 王向阳, 翁信军, 杨祥, 徐学涛, 童晓峰, 方萍, 伍新燕, 梅天胜, 化学学报, 2019, 77, 866.)
doi: 10.6023/A19040135 pmid: 30905151 |
|
|
(t) Zhang, Q.; Liang, K.; Guo, C. CCS Chem. 2021, 3, 338.
doi: 10.31635/ccschem.021.202000720 pmid: 30905151 |
|
| [26] |
(a) Li, H.; Breen, C. P.; Seo, H.; Jamison, T. F.; Fang, Y.-Q.; Bio, M. M. Org. Lett. 2018, 20, 1338.
doi: 10.1021/acs.orglett.8b00070 |
|
(b) Jiao, K.-J.; Li, Z.-M.; Xu, X.-T.; Zhang, L.-P.; Li, Y.-Q.; Zhang, K.; Mei, T.-S. Org. Chem. Front. 2018, 5, 2244.
doi: 10.1039/C8QO00507A |
|
|
(c) Lai, Y.-L.; Huang, J.-M. Org. Lett. 2017, 19, 2022.
doi: 10.1021/acs.orglett.7b00473 |
|
|
(d) Qiu, H.; Shuai, B.; Wang, Y.-Z.; Liu, D.; Chen, Y.-G.; Gao, P.-S.; Ma, H.-X.; Chen, S.; Mei, T.-S. J. Am. Chem. Soc. 2020, 142, 9872.
doi: 10.1021/jacs.9b13117 |
|
| [27] |
(a) Chen, X.; Luo, X.; Peng, X.; Guo, J.; Zai, J.; Wang, P. Chem. Eur. J. 2020, 26, 3226.
doi: 10.1002/chem.v26.15 pmid: 28704055 |
|
(b) Gomes, P.; Gosmini, C.; Périchon, J. J. Org. Chem. 2003, 68, 1142.
doi: 10.1021/jo026421b pmid: 28704055 |
|
|
(c) Gomes, P.; Gosmini, C.; Périchon, J. Tetrahedron 2003, 59, 2999.
doi: 10.1016/S0040-4020(03)00404-6 pmid: 28704055 |
|
|
(d) Jiao, K.-J.; Liu, D.; Ma, H.-X.; Qiu, H.; Fang, P.; Mei, T.-S. Angew. Chem., Int. Ed. 2020, 59, 6520.
doi: 10.1002/anie.v59.16 pmid: 28704055 |
|
|
(e) Kumar, G. S.; Peshkov, A.; Brzozowska, A.; Nikolaienko, P.; Zhu, C.; Rueping, M. Angew. Chem., Int. Ed. 2020, 59, 6513.
doi: 10.1002/anie.v59.16 pmid: 28704055 |
|
|
(f) Wang, X.-X.; Lu, X.; Li, Y.; Wang, J.-W.; Fu, Y. Sci. China Chem. 2020, 63, 1586.
doi: 10.1007/s11426-020-9838-x pmid: 28704055 |
|
|
(g) Truesdell, B. L.; Hamby, T. B.; Sevov, C. S. J. Am. Chem. Soc. 2020, 142, 5884.
doi: 10.1021/jacs.0c01475 pmid: 28704055 |
|
|
(h) Perkins, R. J.; Pedro, D. J.; Hansen, E. C. Org. Lett. 2017, 19, 3755.
doi: 10.1021/acs.orglett.7b01598 pmid: 28704055 |
|
|
(i) Perkins, R. J.; Hughes, A. J.; Weix, D. J.; Hansen, E. C. Org. Process Res. Dev. 2019, 23, 1746.
doi: 10.1021/acs.oprd.9b00232 pmid: 28704055 |
|
| [28] |
Jiang, W.-T.; Yang, S.; Xu, M.-Y.; Xie, X.-Y.; Xiao, B. Chem. Sci. 2020, 11, 488.
doi: 10.1039/C9SC04288A |
| [29] |
(a) Wang, X.-X.; Yu, L.; Lu, X.; Zhang, Z.-L.; Liu, D.-G.; Tian, C.; Fu, Y. CCS Chem. 2021, 3, 727.
pmid: 27033405 |
|
(b) Lu, X.; Xiao, B.; Zhang, Z.; Gong, T.; Su, W.; Yi, J.; Fu, Y.; Liu, L. Nat. Commun. 2016, 7, 11129.
doi: 10.1038/ncomms11129 pmid: 27033405 |
|
|
(c) Wang, J.-W.; Li, Y.; Nie, W.; Chang, Z.; Yu, Z.-A.; Zhao, Y.-F.; Lu, X.; Fu, Y. Nat. Commun. 2021, 12, 1313.
doi: 10.1038/s41467-021-21600-x pmid: 27033405 |
|
| [30] |
Supranovich, V. I.; Levin, V. V.; Kokorekin, V. A.; Dilman, A. D. Adv. Synth. Catal. 2021, 363, 2888.
doi: 10.1002/adsc.v363.11 |
| [1] | 徐利军, 李宗军, 韩福社, 高翔. N,N-二甲基甲酰胺促进的富勒烯稠合噁唑啉衍生物的合成[J]. 有机化学, 2024, 44(1): 242-250. |
| [2] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [3] | 钟赟哲, 陈颖, 俞磊, 周宏伟. 电化学介导羧酸与醇的酯化反应[J]. 有机化学, 2023, 43(8): 2855-2863. |
| [4] | 张周, 郭钰, 羊静, 吴丹, 王佳昕, 洪欣玥, 蔡佩君, 荣良策. 电化学促进咪唑并[1,2-a]吡啶与二氯(溴)乙烷及碘仿的卤化反应[J]. 有机化学, 2023, 43(6): 2104-2109. |
| [5] | 张俊颖, 赵晓静, 李干鹏, 何永辉. 室温下电化学合成保护型有机硼酸RB(dan)[J]. 有机化学, 2023, 43(5): 1815-1823. |
| [6] | 杜琳琳, 张华. 芳烃与烷烃化合物参与的光化学与电化学硼化反应[J]. 有机化学, 2023, 43(5): 1726-1741. |
| [7] | 潘永周, 蒙秀金, 王迎春, 何慕雪. 电化学固定CO2构建羧酸衍生物的研究进展[J]. 有机化学, 2023, 43(4): 1416-1434. |
| [8] | 孙丽, 宋国欣, 韩家乐, 李继玉, 赵月, 杨璐华, 张峰, 赵坤, 毛比明. Morita-Baylis-Hillman加合物和N-羟基邻苯二甲酰亚胺的电化学烯丙基烷基化形成C(sp3)—C(sp3)键[J]. 有机化学, 2023, 43(4): 1574-1583. |
| [9] | 黄嘉为, 李潇漫, 徐亮, 韦玉. α-酮酸与硫酚的电化学脱羧偶联: 一种合成硫代酸酯的新方法[J]. 有机化学, 2023, 43(2): 756-762. |
| [10] | 宋树勇, 徐森苗. 三氟甲基烯烃的选择性C-F键活化最新进展[J]. 有机化学, 2023, 43(2): 411-425. |
| [11] | 魏琬絜, 詹磊, 高雷, 黄国保, 马献力. 电化学合成C-磺酰基化合物的研究进展[J]. 有机化学, 2023, 43(1): 17-35. |
| [12] | 危斌, 周子龙, 秦景灏, 严泽宇, 郭嘉程, 雷澍, 谢叶香, 欧阳旋慧, 宋仁杰. 氧杂蒽与亚磺酸钠的电化学氧化C(sp3)—H磺酰化反应[J]. 有机化学, 2023, 43(1): 186-194. |
| [13] | 李海琼, 尹梦云, 谢芬芬, 张正兵, 韩盼, 敬林海. 通过电化学Appel反应合成腈[J]. 有机化学, 2022, 42(7): 2229-2235. |
| [14] | 顾清云, 程振凤, 曾小宝. 电化学氧化三氟甲基亚磺酸钠与α-羰基二硫缩烯酮的三氟甲基化反应[J]. 有机化学, 2022, 42(5): 1537-1544. |
| [15] | 付拯江, 杨振江, 孙丽, 尹健, 伊学政, 蔡琥, 雷爱文. 无金属条件下亚磺酸钠与酚类化合物形成芳基磺酸酯的电化学合成反应[J]. 有机化学, 2022, 42(2): 600-606. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||