有机化学 ›› 2023, Vol. 43 ›› Issue (1): 186-194.DOI: 10.6023/cjoc202207012 上一篇 下一篇
研究论文
危斌†, 周子龙†, 秦景灏*( ), 严泽宇, 郭嘉程, 雷澍, 谢叶香, 欧阳旋慧*(
), 严泽宇, 郭嘉程, 雷澍, 谢叶香, 欧阳旋慧*( ), 宋仁杰*(
), 宋仁杰*( )
)
收稿日期:2022-07-05
修回日期:2022-07-29
发布日期:2022-09-15
通讯作者:
秦景灏, 欧阳旋慧, 宋仁杰
作者简介:基金资助:
Bin Wei†, Zilong Zhou†, Jinghao Qin( ), Zeyu Yan, Jiacheng Guo, Shu Lei, Yexiang Xie, Xuanhui Ouyang(
), Zeyu Yan, Jiacheng Guo, Shu Lei, Yexiang Xie, Xuanhui Ouyang( ), Renjie Song(
), Renjie Song( )
)
Received:2022-07-05
Revised:2022-07-29
Published:2022-09-15
Contact:
Jinghao Qin, Xuanhui Ouyang, Renjie Song
About author:Supported by:文章分享
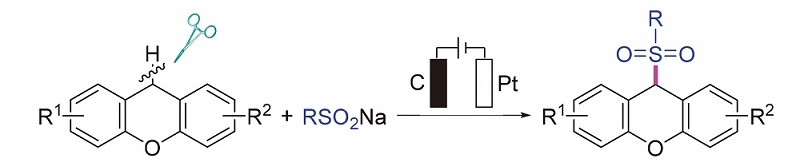
研究了氧杂蒽与亚硫酸钠的C(sp3)—H键的直接电化学磺酰化反应. 在室温下一步合成了多种9-(芳基磺酰基)-9H氧杂蒽和9-(烷基磺酰基)-9H氧杂蒽. 该反应通过自由基途径进行, 并在该电化学磺酰化转化下形成新的C—S键. 这种策略的显著优点包括无需过渡金属和额外的氧化剂、反应条件温和、操作简单、底物范围广以及优异的官能团耐受性.
危斌, 周子龙, 秦景灏, 严泽宇, 郭嘉程, 雷澍, 谢叶香, 欧阳旋慧, 宋仁杰. 氧杂蒽与亚磺酸钠的电化学氧化C(sp3)—H磺酰化反应[J]. 有机化学, 2023, 43(1): 186-194.
Bin Wei, Zilong Zhou, Jinghao Qin, Zeyu Yan, Jiacheng Guo, Shu Lei, Yexiang Xie, Xuanhui Ouyang, Renjie Song. Electrochemical Oxidative C(sp3)—H Sulfonylation of Xanthenes with Sodium Sulfinates[J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 186-194.
| Entry | Variation from the standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 71 |
| 2 | nBu4NPF6 instead of nBu4NBF4 | 52 |
| 3 | TBAI instead of nBu4NBF4 | 41 |
| 4 | TBAB instead of nBu4NBF4 | 57 |
| 5 | Pt(+)/C(–) instead of C(+)/Pt(–) | 59 |
| 6 | C(+)/C(–) instead of C(+)/Pt(–) | 64 |
| 7 | C(+)/RVC(–) instead of C(+)/Pt(–) | 56 |
| 8 | C(+)/Fe(–) instead of C(+)/Pt(–) | 33 |
| 9 | C(+)/Zn(–) instead of C(+)/Pt(–) | 58 |
| 10 | C(+)/Ni(–) instead of C(+)/Pt(–) | 49 |
| 11 | Without AcOH | Trace |
| 12 | HCOOH instead of AcOH | 55 |
| 13 | H3PO4 instead of AcOH | 42 |
| 14 | H3BO3 instead of AcOH | 53 |
| 15 | MsOH instead of AcOH | 45 |
| 16 | AcOH (1 equiv.) | 56 |
| 17 | AcOH (3 equiv.) | 61 |
| 18 | No electric current | 0 |
| 19 | 5 mA instead of 10 mA, 3.5 h | 60 |
| 20 | 15 mA instead of 10 mA, 2 h | 51 |
| 21 | CF3CH2OH instead of HFIP | 38 |
| 22 | CH3CH2OH instead of HFIP | Trace |
| 23 | MeOH instead of HFIP | 42 |
| 24 | MeCN instead of HFIP | 52 |
| 25 | ClCH2CH2Cl instead of HFIP | 44 |
| 26 | MeCN/HFIP (V∶V=1∶1, 5 mL) | 40 |
| 27 | ClCH2CH2Cl/HFIP (V∶V=1∶1, 5 mL) | 45 |
| Entry | Variation from the standard conditions | Yieldb/% |
|---|---|---|
| 1 | None | 71 |
| 2 | nBu4NPF6 instead of nBu4NBF4 | 52 |
| 3 | TBAI instead of nBu4NBF4 | 41 |
| 4 | TBAB instead of nBu4NBF4 | 57 |
| 5 | Pt(+)/C(–) instead of C(+)/Pt(–) | 59 |
| 6 | C(+)/C(–) instead of C(+)/Pt(–) | 64 |
| 7 | C(+)/RVC(–) instead of C(+)/Pt(–) | 56 |
| 8 | C(+)/Fe(–) instead of C(+)/Pt(–) | 33 |
| 9 | C(+)/Zn(–) instead of C(+)/Pt(–) | 58 |
| 10 | C(+)/Ni(–) instead of C(+)/Pt(–) | 49 |
| 11 | Without AcOH | Trace |
| 12 | HCOOH instead of AcOH | 55 |
| 13 | H3PO4 instead of AcOH | 42 |
| 14 | H3BO3 instead of AcOH | 53 |
| 15 | MsOH instead of AcOH | 45 |
| 16 | AcOH (1 equiv.) | 56 |
| 17 | AcOH (3 equiv.) | 61 |
| 18 | No electric current | 0 |
| 19 | 5 mA instead of 10 mA, 3.5 h | 60 |
| 20 | 15 mA instead of 10 mA, 2 h | 51 |
| 21 | CF3CH2OH instead of HFIP | 38 |
| 22 | CH3CH2OH instead of HFIP | Trace |
| 23 | MeOH instead of HFIP | 42 |
| 24 | MeCN instead of HFIP | 52 |
| 25 | ClCH2CH2Cl instead of HFIP | 44 |
| 26 | MeCN/HFIP (V∶V=1∶1, 5 mL) | 40 |
| 27 | ClCH2CH2Cl/HFIP (V∶V=1∶1, 5 mL) | 45 |
| [1] |
(a) Lin, X.-Y.; Yang, Y.-Y.; Zhao, Y.-H.; Fu, Q.-L. Ecotoxicology 2012, 21, 1281.
doi: 10.1007/s10646-012-0882-7 |
|
(b) Foote, K. M.; Blades, K.; Cronin, A.; Fillery, S.; Guichard, S. S.; Hassall, L.; Hickson, I.; Jacq, X.; Jewsbury, P. J.; McGuire, T. M.; Nissink, J. W. M.; Odedra, R.; Page, K.; Perkins, P.; Suleman, A.; Tam, K.; Thommes, P.; Broadhurst, R.; Wood, C. J. Med. Chem. 2013, 56, 2125.
doi: 10.1021/jm301859s |
|
|
(c) Navada, S. C.; Silverman, L. R. Expert Rev. Anticancer Ther. 2016, 16, 805.
doi: 10.1080/14737140.2016.1209413 |
|
|
(d) Takeda, Y.; Kuroki, K.; Chinen, T.; Kitagawa, D. Cell Struct. Funct. 2020, 45, 57.
doi: 10.1247/csf.20007 |
|
| [2] |
(a) Wang, M.; Zhao, J.-Y.; Jiang, X.-F. ChemSusChem 2019, 12, 3064.
doi: 10.1002/cssc.201802919 |
|
(b) Ye, S.-Q.; Zheng, D.-Q.; Wu, J.; Qiu, G. Chem. Commun. 2019, 55, 2214.
doi: 10.1039/C9CC00347A |
|
|
(c) Zhang, J.; Xie, W.-L.; Ye, S.-Q.; Wu, J. Org. Chem. Front. 2019, 6, 2254.
doi: 10.1039/c9qo00520j |
|
|
(d) Chen, S.-H.; Li, Y.-P.; Wang, M.; Jiang, X.-F. Green Chem. 2020, 22, 322.
doi: 10.1039/C9GC03841H |
|
|
(e) Meng, Y.-Y.; Wang, M.; Jiang, X.-F. Angew. Chem., Int. Ed. 2020, 59, 1346.
doi: 10.1002/anie.201911449 |
|
|
(f) Ye, S.-Q.; Zhou, K.-D.; Rojsitthisak, P.; Wu, J. Org. Chem. Front. 2020, 7, 14.
doi: 10.1039/C9QO01274E |
|
| [3] |
(a) Wang, N.-Z.; Saidhareddy, P.; Jiang, X.-F. Nat. Prod. Rep. 2020, 37, 246.
doi: 10.1039/C8NP00093J |
|
(b) Wei, B.; Li, K.-W.; Wu, Y.-C.; Tong, S.-Q.; Song, R.-J. Synthesis 2020, 52, 3855.
doi: 10.1055/s-0040-1707835 |
|
|
(c) Ye, S.-Q.; Li, X.-F.; Xie, W.-L.; Wu, J. Eur. J. Org. Chem. 2020, 2020, 1274.
|
|
|
(d) Tong, S.-Q.; Li, K.-W.; Ouyang, X.-H.; Song, R.-J.; Li, J.-H. Green Synth. Catal. 2021, 2, 145.
|
|
| [4] |
(a) Yamamoto, H.; Nakata, K. Eur. J. Org. Chem. 2019, 2019, 4906.
|
|
(b) Kanyiva, K. S.; Uchida, K.; Shibata, T. Bull. Chem. Soc. Jpn. 2021, 94, 1377.
doi: 10.1246/bcsj.20200393 |
|
|
(c) Wang, X.-L.; Bai, X.; Wu, C.-F.; Dong, Y.-X.; Zhang, M.; Fan, L.-L.; Tang, L.; Yang, Y.-Y.; Zhang, J.-Q. Asian J. Org. Chem. 2021, 10, 386.
doi: 10.1002/ajoc.202000668 |
|
| [5] |
(a) Wei, W.-T.; Zhou, M.-B.; Fan, J.-H.; Liu, W.; Song, R.-J.; Liu, Y.; Hu, M.; Xie, P.; Li, J.-H. Angew. Chem., Int. Ed. 2013, 52, 3638.
doi: 10.1002/anie.201210029 |
|
(b) Zuo, Z.-W.; Ahneman, D. T.; Chu, L.-L.; Terrett, J. A.; Doyle, A. G.; MacMillan, D. W. C. Science 2014, 345, 437.
|
|
|
(c) Tang, S.; Xu, Z.-H.; Liu, T.; Wang, S.-W.; Yu, J.; Liu, J.; Hong, Y.; Chen, S.-L.; He, J.; Li, J.-H. Angew. Chem., Int. Ed. 2021, 60, 21360.
doi: 10.1002/anie.202106273 |
|
| [6] |
(a) Lin, M.-Y.; Xu, K.; Jiang, Y.-Y.; Liu, Y.-G.; Sun, B.-G.; Zeng, C.-C. Adv. Synth. Catal. 2018, 360, 1665.
doi: 10.1002/adsc.201701536 |
|
(b) Li, K.-J.; Jiang, Y.-Y.; Xu, K.; Zeng, C.-C.; Sun, B.-G. Green Chem. 2019, 21, 4412.
doi: 10.1039/C9GC01474H |
|
|
(c) Yang, Y.-Z.; Song, R.-J.; Li, J.-H. Org. Lett. 2019, 21, 3228.
doi: 10.1021/acs.orglett.9b00947 |
|
|
(d) Yang, Y.-Z.; Wu, Y.-C.; Song, R.-J.; Li, J.-H. Chem. Commun. 2020, 56, 7585.
doi: 10.1039/D0CC02580A |
|
|
(e) Wei, B.; Qin, J.-H.; Yang, Y.-Z.; Xie, Y.-X.; Ouyang, X.-H.; Song, R.-J. Org. Chem. Front. 2022, 9, 816.
doi: 10.1039/D1QO01714D |
|
| [7] |
(a) Xie, Y.-Y.; Wang, Y.-C.; He, Y.; Hu, D.-C.; Wang, H.-S.; Pan, Y.-M. Green Chem. 2017, 19, 656.
doi: 10.1039/C6GC01553K |
|
(b) Wang, Y.-C.; Liu, L.-Q.; Wang, G.-M.; Ouyang, H.; Li, Y.-J. Green Chem. 2018, 20, 604.
doi: 10.1039/C7GC03267F |
|
|
(c) Wu, Y.-C.; Jiang, S.-S.; Song, R.-J.; Li, J.-H. Chem. Commun. 2019, 55, 4371.
doi: 10.1039/C9CC01332F |
|
|
(d) Qin, J.-H.; Luo, M.-J.; An, D.-L.; Li, J.-H. Angew. Chem., Int. Ed. 2020, 60, 1861.
doi: 10.1002/anie.202011657 |
|
|
(e) Li, J.; Zhang, S.; Xu, K. Chin. Chem. Lett. 2021, 32, 2729.
doi: 10.1016/j.cclet.2021.03.027 |
|
| [8] |
Wei, W.-J.; Zhong, Y.-J.; Feng, Y.-F.; Gao, L.; Tang, H.-T.; Pan, Y.-M.; Ma, X.-L.; Mo, Z.-Y. Adv. Synth. Catal. 2022, 364, 726.
doi: 10.1002/adsc.202101289 |
| [9] |
CCDC 2131087 (3aa) contain the supplementary crystallo-graphic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
|
| [10] |
(a) Luo, M.-J.; Liu, B.; Li, Y.; Hu, M.; Li, J.-H. Adv. Synth. Catal. 2019, 361, 1538.
doi: 10.1002/adsc.201801492 |
|
(b) Wu, Y.-C.; Jiang, S.-S.; Luo, S.-Z.; Song, R.-J.; Li, J.-H. Chem. Commun. 2019, 55, 8995.
doi: 10.1039/C9CC03789F |
| [1] | 陈雯雯, 张琴, 张松月, 黄芳芳, 张馨尹, 贾建峰. 无光催化剂条件下可见光诱导炔基碘和亚磺酸钠偶联反应[J]. 有机化学, 2024, 44(2): 584-592. |
| [2] | Yasir Mumtaz, 刘杰, 黄鑫. 铜促进的苯胺类化合物与CF3SO2Na的三氟甲硫基化反应[J]. 有机化学, 2023, 43(2): 679-685. |
| [3] | 吴宇恒, 颜岩, 寮渭巍. 双功能二氧化硫替代物在合成磺酰类化合物中的研究进展[J]. 有机化学, 2023, 43(11): 3713-3727. |
| [4] | 许力, 吕兰兰, 王香善. 铜催化烯醇硅醚与芳基亚磺酸钠合成β-酮砜的研究[J]. 有机化学, 2023, 43(10): 3644-3651. |
| [5] | 王川川, 马志伟, 侯学会, 杨龙华, 陈亚静. N-Ts氰胺在有机合成中的研究与应用[J]. 有机化学, 2023, 43(1): 74-93. |
| [6] | 魏琬絜, 詹磊, 高雷, 黄国保, 马献力. 电化学合成C-磺酰基化合物的研究进展[J]. 有机化学, 2023, 43(1): 17-35. |
| [7] | 徐琳琳, 兰美君, 张慕雨, 张永琪, 冯宇豪, 荣良策, 张金鹏. 芳基乙烯β-H区域选择性三氟甲基磺酰化反应[J]. 有机化学, 2022, 42(7): 2134-2139. |
| [8] | 锅小龙, 王玉贤, 赵志强, 王庆, 左剑, 王陆瑶. 电化学氧化下喹喔啉-2(1H)-酮的三氟甲基化及电描述符对反应性能的评价[J]. 有机化学, 2022, 42(2): 641-649. |
| [9] | 冯易浇, 何静, 韦玥婷, 汤婷, 李春天, 刘平. 一锅两步策略高效合成3-芳基-4-(芳硫基)-1H-吡唑-5-胺衍生物[J]. 有机化学, 2022, 42(1): 226-234. |
| [10] | 任尚峰, 王玉超, 刘晋彪, 邱观音生. N-羟乙基-N-芳基丙炔酰胺的芳基磺酰化及螺-三环化反应[J]. 有机化学, 2021, 41(9): 3652-3659. |
| [11] | 易荣楠, 刘冬娴, 贺江南, 赵明明, 许新华. 过硫酸铵促进喹喔啉-2(1H)-酮三氟甲基化反应[J]. 有机化学, 2021, 41(8): 3285-3291. |
| [12] | 熊云奎, 张健叶, 申裙, 黄嘉宇, 王涛. 苯亚磺酸钠和叔胺电化学耦合生成β-氨基乙烯砜[J]. 有机化学, 2021, 41(7): 2735-2742. |
| [13] | 刘丽, 肖洪, 肖福红, 谢艳军, 黄华文, 邓国军. 亚磺酸钠盐和芳基乙酮/茚酮合成β-酮砜[J]. 有机化学, 2021, 41(12): 4749-4757. |
| [14] | 周翔, 余茹鉴, 王金涛, 廖向文, 熊艳师. 铜催化亚磺酸钠盐参与的萘胺远程磺酰化反应[J]. 有机化学, 2021, 41(11): 4370-4377. |
| [15] | 周鹏, 冯尚伟, 邱会华, 张建涛. 对甲苯亚磺酸钠/KI介导端炔的需氧氧化碘代反应合成1-碘代炔和1,3-二炔[J]. 有机化学, 2021, 41(1): 394-399. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||