有机化学 ›› 2022, Vol. 42 ›› Issue (11): 3562-3587.DOI: 10.6023/cjoc202204060 上一篇 下一篇
所属专题: 有机氟化学虚拟合辑
综述与进展
郭檬檬a, 于子伦a, 陈玉兰a, 葛丹华a,*( ), 马猛涛b, 沈志良a, 褚雪强a,*(
), 马猛涛b, 沈志良a, 褚雪强a,*( )
)
收稿日期:2022-04-25
修回日期:2022-06-24
发布日期:2022-07-13
通讯作者:
葛丹华, 褚雪强
作者简介:基金资助:
Mengmeng Guoa, Zilun Yua, Yulan Chena, Danhua Gea( ), Mengtao Mab, Zhiliang Shena, Xueqiang Chua(
), Mengtao Mab, Zhiliang Shena, Xueqiang Chua( )
)
Received:2022-04-25
Revised:2022-06-24
Published:2022-07-13
Contact:
Danhua Ge, Xueqiang Chu
About author:Supported by:文章分享

与非氟化合物相比, 有机氟化合物由于其特殊的化学和物理性质被广泛应用于药物开发、临床医学、农业化学、材料科学和有机合成等领域. 在过去的几十年中, 在选择性氟化和氟烷基化合成结构多样的含氟分子方面取得了重大进展. 其中, α-氟烷基化羰基代表了生物活性和药物分子中一类重要的分支骨架. 在此背景下, 基于使用二氟烯醇硅醚(DFSEE)作为独特的氟代烷基化试剂, 已经实现了多种引入偕二氟烷基化羰基片段的有效方法, 包括羟醛反应、曼尼希反应、芳基化反应、烯丙基化反应、质子化反应、卤化反应、共轭加成反应和烯化反应等. 另一方面, DFSEE凭借其优异的反应灵活性, 还能与自由基型二氟烷基化和级联反应等新型反应模式相融合. 此外, 还报道了DFSEE参与的O位选择性加成反应, 能够构建多功能的偕二氟烯烃. 鉴于有机氟化合物的重要性和基于二氟烯醇硅醚作为反应物参与合成含氟化合物途径的巨大潜力, 重点介绍了DFSEE作为关键含氟功能化砌块合成氟化物的最新研究进展.
郭檬檬, 于子伦, 陈玉兰, 葛丹华, 马猛涛, 沈志良, 褚雪强. 二氟烯醇硅醚作为含氟砌块在构建有机氟化物中的研究进展[J]. 有机化学, 2022, 42(11): 3562-3587.
Mengmeng Guo, Zilun Yu, Yulan Chen, Danhua Ge, Mengtao Ma, Zhiliang Shen, Xueqiang Chu. Difluorinated Silyl Enol Ethers as Fluorine-Containing Building Blocks for the Synthesis of Organofluorine Compounds[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3562-3587.













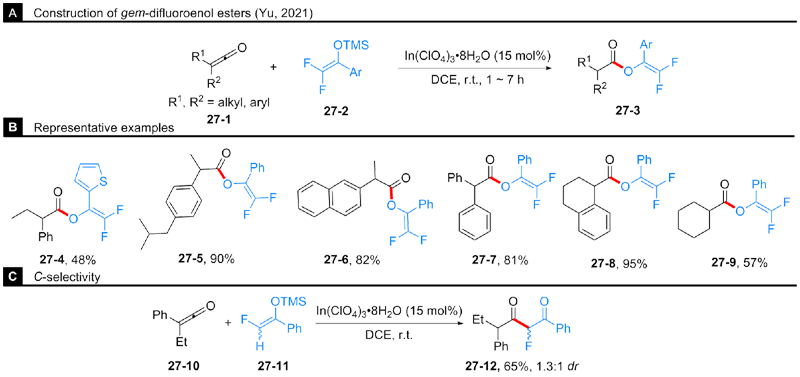
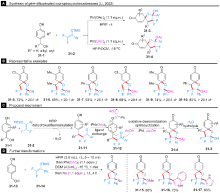

| [1] |
(a) Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
doi: 10.1021/cr4002879 pmid: 25078138 |
|
(b) Prchalová, E.; Štěpánek, O.; Smrček, S.; Kotora, M. Future Med. Chem. 2014, 6, 1201.
doi: 10.4155/fmc.14.53 pmid: 25078138 |
|
| [2] |
Hu, J.; Ding, K. Acta Chim. Sinica 2018, 76, 905. (in Chinese)
doi: 10.6023/A1812E001 |
|
( 胡金波, 丁奎岭, 化学学报 2018, 76, 905.)
doi: 10.6023/A1812E001 |
|
| [3] |
(a) Ge, D.; Chu, X.-Q. Org. Chem. Front. 2022, 9, 2013.
doi: 10.1039/D1QO01749G |
|
(b) Chen, B.; Vicic, D. A. Top. Organomet. Chem. 2014, 52, 113.
|
|
| [4] |
(a) Schirlin, D.; Tarnus, C.; Baltzer, S.; Remy, J. M. Bioorg. Med. Chem. Lett. 1992, 2, 651.
doi: 10.1016/S0960-894X(00)80383-2 pmid: 8551523 |
|
(b) Dreyer, G. B.; Metcalf, B. W. Tetrahedron Lett. 1988, 29, 6885.
doi: 10.1016/S0040-4039(00)88466-X pmid: 8551523 |
|
|
(c) Nikolaou, A.; Kokotou, M. G.; Vasilakaki, S.; Kokotos, G. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2019, 1864, 941.
doi: 10.1016/j.bbalip.2018.08.009 pmid: 8551523 |
|
|
(d) Cregge, R. J.; Durham, S. L.; Farr, R. A.; Gallion, S. L.; Hare, C. M.; Hoffman, R. V.; Janusz, M. J.; Kim, H.-O.; Koehl, J. R.; Mehdi, S.; Metz, W. A.; Peet, N. P.; Pelton, J. T.; Schreuder, H. A.; Sunder, S.; Tardif, C. J. Med. Chem. 1998, 41, 2461.
pmid: 8551523 |
|
|
(e) Silva, A. M.; Cachau, R. E.; Sham, H. L.; Erickson, J. W. J. Mol. Biol. 1996, 255, 321.
pmid: 8551523 |
|
|
(f) Fah, C.; Hardegger, L. A.; Baitsch, L.; Schweizer, W. B.; Meyer, S.; Bur, D.; Diederich, F. Org. Biomol. Chem. 2009, 7, 3947.
doi: 10.1039/b908489d pmid: 8551523 |
|
| [5] |
(a) Mei, H.; Liu, J.; Fustero, S.; Román, R.; Ruzziconi, R.; Soloshonok, V. A.; Han, J. Org. Biomol. Chem. 2019, 17, 762.
doi: 10.1039/C8OB02843E |
|
(b) Gong, Y.; Yu, J.-S.; Hao, Y.-J.; Zhou, Y.; Zhou, J. Asian J. Org. Chem. 2019, 8, 610.
doi: 10.1002/ajoc.201900071 |
|
|
(c) Dong, D.-Q.; Yang, H.; Shi, J.-L.; Si, W.-J.; Wang, Z.-L.; Xu, X.-M. Org. Chem. Front. 2020, 7, 2538.
doi: 10.1039/D0QO00567C |
|
|
(d) Belhomme, M.-C.; Besset, T.; Poisson, T.; Pannecoucke, X. Chem. Eur. J. 2015, 21, 12836.
doi: 10.1002/chem.201501475 |
|
|
(e) Rong, J.; Ni, C.; Hu, J. Asian J. Org. Chem. 2017, 6, 139.
doi: 10.1002/ajoc.201600509 |
|
| [6] |
Yamana, M.; Ishihara, T.; Ando, T. Tetrahedron Lett. 1983, 24, 507.
doi: 10.1016/S0040-4039(00)81449-5 |
| [7] |
(a) Liu, Y.-L.; Zhou, J. Chem. Commun. 2012, 48, 1919.
doi: 10.1039/c2cc17140f |
|
(b) Liu, Y.-L.; Zeng, X.-P.; Zhou, J. Acta Chim. Sinica 2012, 70, 1451 (in Chinese)
doi: 10.6023/A12040145 |
|
|
( 刘运林, 周剑, 化学学报 2012, 70, 1451.)
doi: 10.6023/A12040145 |
|
|
(c) Liu, Y.-L.; Liao, F.-M.; Niu, Y.-F.; Zhao, X.-L.; Zhou, J. Org. Chem. Front. 2014, 1, 742.
doi: 10.1039/C4QO00126E |
|
|
(d) Liao, F.-M.; Liu, Y.-L.; Yu, J.-S.; Zhou, F.; Zhou, J. Org. Biomol. Chem. 2015, 13, 8906.
doi: 10.1039/C5OB01125F |
|
|
(e) Liao, F.-M.; Gao, X.-T.; Hu, X.-S.; Xie, S.-L.; Zhou, J. Sci. Bull. 2017, 62, 1504.
doi: 10.1016/j.scib.2017.10.016 |
|
| [8] |
(a) Yu, J.-S.; Zhou, J. Org. Biomol. Chem. 2015, 13, 10968.
doi: 10.1039/C5OB01895A |
|
(b) Yu, J.-S.; Zhou, J. Org. Chem. Front. 2016, 3, 298.
doi: 10.1039/C5QO00407A |
|
|
(c) Hu, X.-S.; Du, Y.; Yu, J.-S.; Liao, F.-M.; Ding, P.-G.; Zhou, J. Synlett 2017, 28, 2194.
doi: 10.1055/s-0036-1588475 |
|
|
(d) Hu, X.-S.; Yu, J.-S.; Gong, Y.; Zhou, J. J. Fluorine Chem. 2019, 219, 106.
doi: 10.1016/j.jfluchem.2019.01.003 |
|
| [9] |
(a) Uneyama, K.; Tanaka, H.; Kobayashi, S.; Shioyama, M.; Amii, H. Org. Lett. 2004, 6, 2733.
pmid: 15281756 |
|
(b) Guo, Y.; Shreeve, J. M. Chem. Commun. 2007, 3583.
pmid: 15281756 |
|
|
(c) Guo, Y.; Twamley, B.; Shreeve, J. M. Org. Biomol. Chem. 2009, 7, 1716.
doi: 10.1039/b900311h pmid: 15281756 |
|
|
(d) Guo, Y.; Tao, G.-H.; Blumenfeld, A.; Shreeve, J. M. Organometallics 2010, 29, 1818.
doi: 10.1021/om1000259 pmid: 15281756 |
|
| [10] |
(a) Lefebvre, O.; Brigaud, T.; Portella, C. J. Org. Chem. 2001, 66, 4348.
pmid: 11397175 |
|
(b) Belanger, E.; Cantin, K.; Messe, O.; Tremblay, M.; Paquin, J.-F. J. Am. Chem. Soc. 2007, 129, 1034.
doi: 10.1021/ja067501q pmid: 11397175 |
|
| [11] |
(a) Poisson, T.; Gembus, V.; Dalla, V.; Oudeyer, S.; Levacher, V. J. Org. Chem. 2010, 75, 7704.
doi: 10.1021/jo101585t pmid: 20958068 |
|
(b) Liao, K.; Hu, X.-S.; Zhu, R.-Y.; Rao, R.-H.; Yu, J.-S.; Zhou, F.; Zhou, J. Chin. J. Chem. 2019, 37, 799.
doi: 10.1002/cjoc.201900198 pmid: 20958068 |
|
| [12] |
Higashiya, S.; Chung, W. J.; Lim, D. S.; Ngo, S. C.; Kelly IV, W. H.; Toscano, P. J.; Welch, J. T. J. Org. Chem. 2004, 69, 6323.
doi: 10.1021/jo049551o |
| [13] |
(a) Yu, J.-S.; Liao, F.-M.; Gao, W.-M.; Liao, K.; Zuo, R.-L.; Zhou, J. Angew. Chem., Int. Ed. 2015, 54, 7381.
doi: 10.1002/anie.201501747 |
|
(b) Hao, Y.-J.; Hu, X.-S.; Yu, J.-S.; Zhou, F.; Zhou, Y.; Zhou, J. Tetrahedron 2018, 74, 7395.
doi: 10.1016/j.tet.2018.11.017 |
|
| [14] |
(a) Liao, F.-M.; Cao, Z.-Y.; Yu, J.-S.; Zhou, J. Angew. Chem., Int. Ed. 2017, 56, 2459.
doi: 10.1002/anie.201611625 |
|
(b) Liao, F.-M.; Du, Y.; Zhou, F.; Zhou, J. Acta Chim. Sinica 2018, 76, 862. (in Chinese)
doi: 10.6023/A18060238 |
|
|
( 廖富民, 杜溢, 周锋, 周剑, 化学学报 2018, 76, 862.)
doi: 10.6023/A18060238 |
|
| [15] |
Hu, X.-S.; Yu, J.-S.; Zhou, J. Chem. Commun. 2019, 55, 13638.
doi: 10.1039/C9CC07677H |
| [16] |
Chu, X.-Q.; Ge, D.; Cui, Y.-Y.; Shen, Z.-L.; Li, C.-J. Chem. Rev. 2021, 121, 12548.
doi: 10.1021/acs.chemrev.1c00084 |
| [17] |
Yu, J.; Liu, Y.; Tang, J.; Wang X.; Zhou, J. Angew. Chem., Int. Ed. 2014, 53, 9512.
doi: 10.1002/anie.201404432 |
| [18] |
(a) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
doi: 10.1126/science.1131943 pmid: 17901324 |
|
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
doi: 10.1039/B610213C pmid: 17901324 |
|
|
(c) O’Hagan, D. Chem. Soc. Rev. 2008, 37, 308.
doi: 10.1039/B711844A pmid: 17901324 |
|
| [19] |
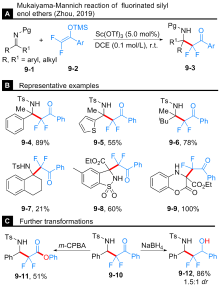
Tian, Y.-P.; Gong, Y.; Hu, X.-S.; Yu, J.-S.; Zhou, Y.; Zhou, J. Org. Biomol. Chem. 2019, 17, 9430.
doi: 10.1039/C9OB02129A |
| [20] |
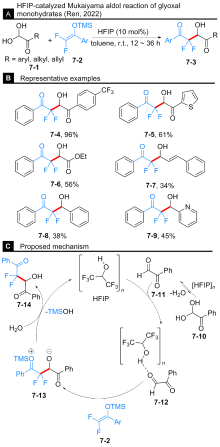
Yang, J.; Liu, S.; Hong, P.; Li, J.; Wang, Z.; Ren, J. J. Org. Chem. 2022, 87, 1144.
doi: 10.1021/acs.joc.1c02504 |
| [21] |
(a) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320.
doi: 10.1039/B610213C |
|
(b) Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. Synthesis 2013, 45, 1.
doi: 10.1055/s-0032-1317575 |
|
| [22] |
Liu, S.; Li, Y.; Wang, F.; Ma, C.; Yang, G.; Yang, J.; Ren, J. Synthesis 2022, 54, 161.
doi: 10.1055/a-1581-2408 |
| [23] |
(a) Jonet, S.; Cherouvrier, F.; Brigaud, T.; Portella, C. Eur. J. Org. Chem. 2005, 4304.
|
|
(b) Yuan, Z.; Wei, Y.; Shi, M. Chin. J. Chem. 2010, 28, 1709.
doi: 10.1002/cjoc.201090289 |
|
|
(c) Chu, L.; Zhang, X.; Qing, F.-L. Org. Lett. 2009, 11, 2197.
doi: 10.1021/ol900541n |
|
|
(d) Wu, Y.-B.; Wan, L.; Lu, G.-P.; Cai, C. Eur. J. Org. Chem. 2017, 3438.
|
|
| [24] |
Hu, X.-S.; Ding, P.-G.; Yu, J.-S.; Zhou, J. Org. Chem. Front. 2019, 6, 2500.
doi: 10.1039/C9QO00577C |
| [25] |
(a) Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328.
doi: 10.1021/acs.chemrev.5b00547 pmid: 18986207 |
|
(b) Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299.
doi: 10.1021/cr800332c pmid: 18986207 |
|
| [26] |
Huang, X.; Zhang, Y.; Zhang, C.; Zhang, L.; Xu, Y.; Kong, L.; Wang, Z.-X.; Peng, B. Angew. Chem., Int. Ed. 2019, 58, 5956.
doi: 10.1002/anie.201900745 |
| [27] |
(a) Roche, S. P.; Porco Jr, J. A. Angew. Chem., Int. Ed. 2011, 50, 4068.
doi: 10.1002/anie.201006017 pmid: 30839047 |
|
(b) Zheng, C.; You, S.-L. Nat. Prod. Rep. 2019, 36, 1589.
doi: 10.1039/c8np00098k pmid: 30839047 |
|
|
(c) Ding, Q.; Ye, Y.; Fan, R. Synthesis 2013, 45, 1.
doi: 10.1055/s-0032-1317575 pmid: 30839047 |
|
| [28] |
Huang, X.; Zhang, Y.; Liang, W.; Zhang, Q.; Zhan, Y.; Kong, L.; Peng, B. Chem. Sci. 2020, 11, 3048.
doi: 10.1039/d0sc00244e pmid: 34122809 |
| [29] |
(a) Ilardi, E. A.; Vitaku, E.; Njardarson, J. T. J. Med. Chem. 2014, 57, 2832.
doi: 10.1021/jm401375q |
|
(b) Feng, M.; Tang, B.; Liang, S. H.; Jiang, X. Curr. Top. Med. Chem. 2016, 16, 1200.
doi: 10.2174/1568026615666150915111741 |
|
| [30] |
Huang, X.; Zhao, W.; Liang, Y.; Wang, M.; Zhan, Y.; Zhang, Y.; Kong, L.; Wang, Z.-X.; Peng, B. Org. Chem. Front. 2021, 8, 1280.
doi: 10.1039/D0QO01513J |
| [31] |
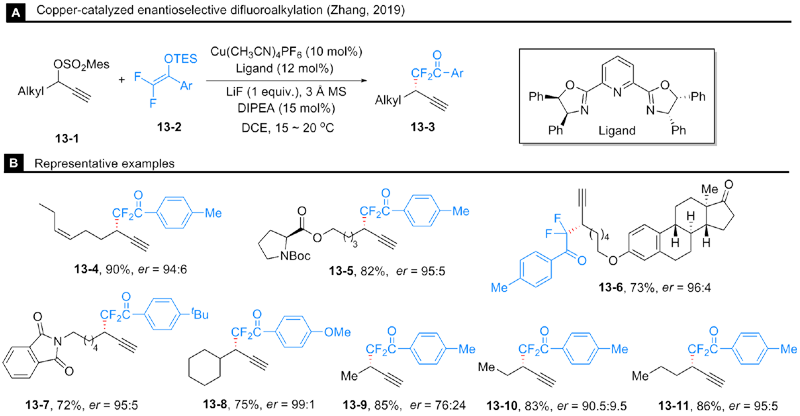
Gao, X.; Cheng, R.; Xiao, Y.-L.; Wan, X.-L.; Zhang, X. Chem 2019, 5, 2987.
doi: 10.1016/j.chempr.2019.09.012 |
| [32] |
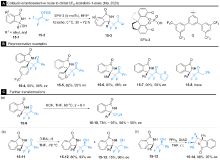
Jiang, X.; Meyer, D.; Baran, D.; Cortes Gonzalez, M. A.; Szabo, K. J. J. Org. Chem. 2020, 85, 8311.
doi: 10.1021/acs.joc.0c01030 |
| [33] |
(a) Speck, K.; Magauer, T. J. Org. Chem. 2013, 9, 2048.
|
|
(b) Bhatia, R. K. Curr. Top. Med. Chem. 2016, 17, 189.
doi: 10.2174/1568026616666160530154100 |
|
| [34] |
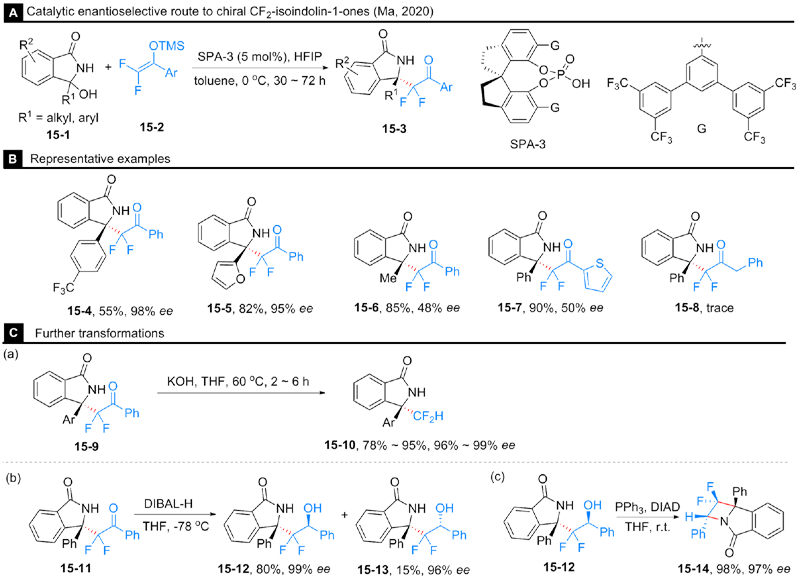
Rong, M.-Y.; Li, J.-S.; Zhou, Y.; Zhang, F.-G.; Ma, J.-A. Org. Lett. 2020, 22, 9010.
doi: 10.1021/acs.orglett.0c03406 |
| [35] |
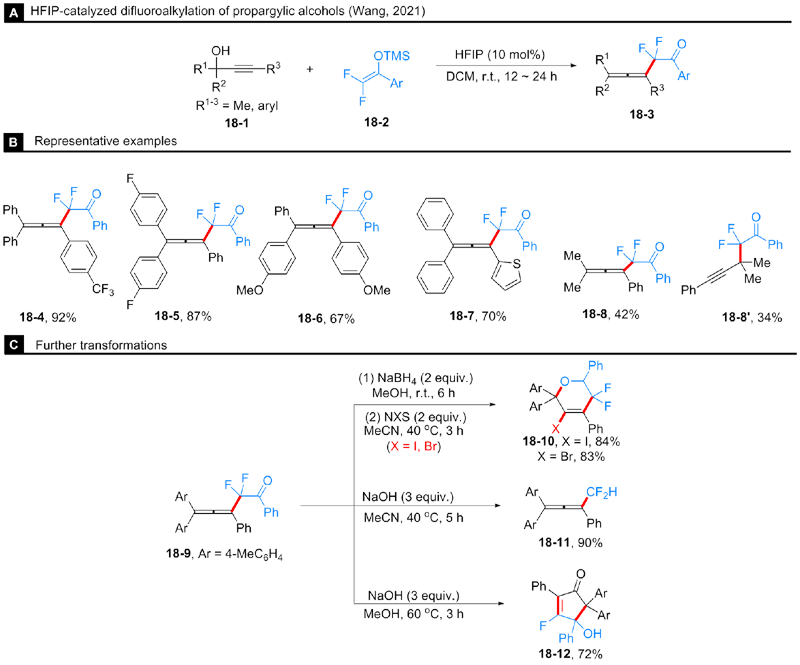
Hao, Y.-J.; Gong, Y.; Zhou, Y.; Zhou, J.; Yu, J.-S. Org. Lett. 2020, 22, 8516.
doi: 10.1021/acs.orglett.0c03123 |
| [36] |
(a) Wencel-Delord, J.; Colobert, F. Org. Chem. Front. 2016, 3, 394.
doi: 10.1039/C5QO00398A |
|
(b) Colomer, I.; Chamberlain, A. E. R.; Haughey, M. B.; Donohoe, T. J. Nat. Rev. Chem. 2017, 1, 0088.
doi: 10.1038/s41570-017-0088 |
|
| [37] |
Li, J.; Xi, W.; Zhong, R.; Yang, J.; Wang, L.; Ding, H.; Wang, Z. Chem. Commun. 2021, 57, 1050.
doi: 10.1039/D0CC06980A |
| [38] |
(a) Zhang, K. F.; Bian, K. J.; Li, C.; Sheng, J.; Li, Y.; Wang, X. S. Angew. Chem., Int. Ed. 2019, 58, 5069.
doi: 10.1002/anie.201813184 pmid: 31964875 |
|
(b) Taj Muhammad, M.; Jiao, Y.; Ye, C.; Chiou, M.-F.; Israr, M.; Zhu, X.; Li, Y.; Wen, Z.; Studer, A.; Bao, H. Nat. Commun. 2020, 11, 416.
doi: 10.1038/s41467-019-14254-3 pmid: 31964875 |
|
| [39] |
Li, J.; Xi, W.; Liu, S.; Ruan, C.; Zheng, X.; Yang, J.; Wang, L.; Wang, Z. Org. Lett. 2021, 23, 7264.
doi: 10.1021/acs.orglett.1c02659 |
| [40] |
(a) Hu, X.-S.; He, J.-X.; Dong, S.-Z.; Zhao, Q.-H.; Yu, J.-S.; Zhou, J. Nat. Commun. 2020, 11, 5500.
doi: 10.1038/s41467-020-19387-4 |
|
(b) Chen, G.; Liu, Y. Chin. J. Org. Chem. 2021, 41, 869. (in Chinese)
doi: 10.6023/cjoc202100015 |
|
|
( 陈国术, 刘运林, 有机化学 2021, 41, 869.)
doi: 10.6023/cjoc202100015 |
|
| [41] |
Li, J.; Liu, S.; Zhong, R.; Yang, Y.; He, Y.; Yang, J.; Ma, Y.; Wang, Z. Org. Lett. 2021, 23, 5859.
doi: 10.1021/acs.orglett.1c01993 |
| [42] |
(a) Hari, D. P.; Schroll, P.; Konig, B. J. Am. Chem. Soc. 2012, 134, 2958.
doi: 10.1021/ja212099r |
|
(b) Bu, M. J.; Niu, T. F.; Cai, C. Catal. Sci. Technol. 2015, 5, 830.
doi: 10.1039/C4CY01523A |
|
| [43] |
Wu, Y.-B.; Lu, G.-P.; Zhou, B.-J.; Bu, M.-J.; Wan, L.; Cai, C. Chem. Commun. 2016, 52, 5965.
doi: 10.1039/C6CC00177G |
| [44] |
Klauck, F. J. R.; James, M. J.; Glorius, F. Angew. Chem., Int. Ed. 2017, 56, 12336.
doi: 10.1002/anie.201706896 |
| [45] |
(a) Huang, Y.; Jia, J.; Huang, Q.-P.; Zhao, L.; Wang, P.; Gu, J.; He, C.-Y. Chem. Commun. 2020, 56, 14247.
doi: 10.1039/D0CC05725H |
|
(b) Huang, Q.-P.; Huang, Y.; Wang, A.-J.; Zhao, L.; Jia, J.; Yu, Y.; Tong, J.; Gu, J.; He, C.-Y. Org. Chem. Front. 2021, 8, 4438.
doi: 10.1039/D1QO00507C |
|
| [46] |
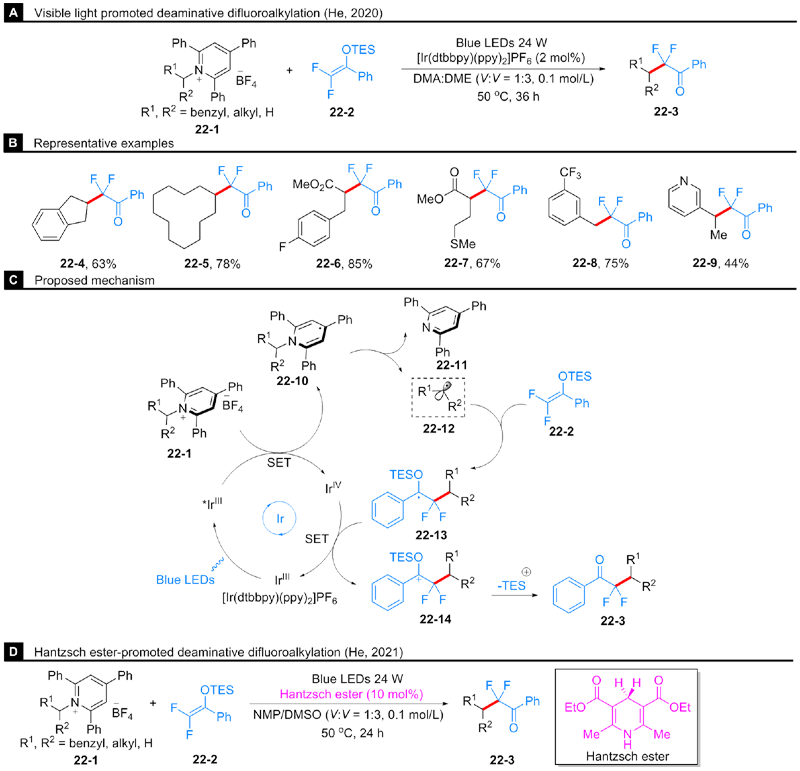
He, F.-S.; Yao, Y.; Xie, W.; Wu, J. Chem. Commun. 2020, 56, 9469.
doi: 10.1039/D0CC03591B |
| [47] |
Song, H.; Cheng, R.; Min, Q.-Q.; Zhang, X. Org. Lett. 2020, 22, 7747.
doi: 10.1021/acs.orglett.0c02997 |
| [48] |
Zhu, X.; Huang, Y.; Xu, X.; Qing, F. Chin. Chem. Lett. 2022, 33, 817.
doi: 10.1016/j.cclet.2021.07.030 |
| [49] |
(a) Borth, P. W.; J. Econ. Entomol. 1986, 79, 1632.
doi: 10.1093/jee/79.6.1632 |
|
(b) Medebielle, M.; Ait-Mohand, S.; Burkhloder, C.; Dolbier, W. R.; Laumond, G.; Aubertin, A. M. J. Fluorine Chem. 2005, 126, 533.
doi: 10.1016/j.jfluchem.2004.12.016 |
|
| [50] |
(a) Zou, J.-Y.; Wang, Y.-Z.; Sun, W.-H.; Lin, W.-J.; Liu, X.-Y. Org. Biomol. Chem. 2021, 19, 8696.
doi: 10.1039/D1OB01530C |
|
(b) Huang, Q.-P.; Li, W.-P.; Li, R.; Zhao, L.; Wang, H.-Y.; Li, X.; Wang, P.; He, C.-Y. Tetrahedron Lett. 2022, 97, 153782.
doi: 10.1016/j.tetlet.2022.153782 |
|
| [51] |
Shi, Y.; Pan, B.-W.; He, J.-X.; Zhou, Y.; Zhou, J.; Yu, J.-S. J. Org. Chem. 2021, 86, 7797.
doi: 10.1021/acs.joc.1c00570 |
| [52] |
((a) Orsi, D. L.; Altman, R. A. Chem. Commun. 2017, 53, 7168.
doi: 10.1039/C7CC02341C |
| [53] |
((a) Hao, Y.-J..; Yu, J.-S..; Zhou, Y..; Wang, X..; Zhou, J.. Acta Chim. Sinica 2018, 76, 925. (in Chinese)
doi: 10.6023/A18080360 |
|
( 郝永佳, 余金生, 周英, 王欣, 周剑, 化学学报 2018, 76, 925.)
doi: 10.6023/A18080360 |
|
| [54] |
He, J.-X.; Zhang, Z.-H.; Mu, B.-S.; Cui, X.-Y.; Zhou, J.; Yu, J.-S. J. Org. Chem. 2021, 86, 9206.
doi: 10.1021/acs.joc.1c00754 |
| [55] |
Li, J.; Liu, S.; Zhong, R.; Yang, Y.; Xu, J.; Yang, J.; Ding, H.; Wang, Z. Org. Lett. 2021, 23, 9526.
doi: 10.1021/acs.orglett.1c03745 |
| [56] |
Li, J.; Xi, W.; Liu, S.; Yang, Y.; Yang, J.; Ding, H.; Wang, Z. Chin. Chem. Lett. 2022, 3007.
|
| [57] |
Rios, R. Chem. Soc. Rev. 2012, 41, 1060.
doi: 10.1039/C1CS15156H |
| [58] |
Wu, H.; Hong, P.; Xi, W.; Li, J. Org. Lett. 2022, 24, 2488.
doi: 10.1021/acs.orglett.2c00550 |
| [1] | 冯莹珂, 王贺, 崔梦行, 孙然, 王欣, 陈阳, 李蕾. 可见光诱导的新型官能化芳基异腈化合物的二氟烷基化环化反应[J]. 有机化学, 2023, 43(8): 2913-2925. |
| [2] | 赵金晓, 魏彤辉, 柯森, 李毅. 可见光催化合成二氟烷基取代的多环吲哚化合物[J]. 有机化学, 2023, 43(3): 1102-1114. |
| [3] | 涂志, 余金生, 周剑. 溴二氟甲基三甲基硅烷的合成及其在有机合成中的应用[J]. 有机化学, 2023, 43(10): 3491-3507. |
| [4] | 孙奇, 孙泽颖, 俞泽, 王光伟. 镍催化炔烃的立体选择性芳基-二氟烷基化反应[J]. 有机化学, 2022, 42(8): 2515-2520. |
| [5] | 程步清, 葛丹华, 汪欣, 褚雪强. 全氟烷基卤化物作为含氟砌块在构建氟烷基取代杂环化合物中的研究进展[J]. 有机化学, 2021, 41(5): 1925-1938. |
| [6] | 朱文庆, 许婷怡, 韩文勇. 二氟甲基重氮甲烷作为含氟砌块的应用研究进展[J]. 有机化学, 2021, 41(4): 1275-1287. |
| [7] | 潘军, 吴晶晶, 吴范宏. 多组分参与的氟烷基化反应研究进展[J]. 有机化学, 2021, 41(3): 983-1001. |
| [8] | 黄帅帅, 聂一雪, 杨晶晶, 郑战江, 曹建, 徐征, 徐利文. 铜催化二氟乙醇的芳基醚化反应及其机理研究[J]. 有机化学, 2020, 40(7): 2018-2025. |
| [9] | 崔丽媛, 陈煌冠, 马静怡, 韩建伟, 王利民. 邻三氟甲磺酸酯基取代二芳基碘盐的合成及其抑菌活性研究[J]. 有机化学, 2019, 39(1): 270-276. |
| [10] | 张柯, 徐修华, 卿凤翎. 直接三氟甲硫基化反应研究进展[J]. 有机化学, 2015, 35(3): 556-569. |
| [11] | 卿凤翎. 从三氟甲基化反应的近年进展看有机氟化学的发展趋势[J]. 有机化学, 2012, 32(05): 815-824. |
| [12] | 熊英, 琚振华, 王晓光, 方向, 吴范宏. 含氟内酯化合物的合成方法研究进展[J]. 有机化学, 2009, 29(11): 1728-1743. |
| [13] | 陈 阳a; 水声霞a ; 方 向a ; 吴范宏*,a,b. β-烷氧乙烯基三氟甲基酮类化合物在有机合成中的应用[J]. 有机化学, 2008, 28(12): 2023-2038. |
| [14] | 曾卓, 廖子英,唐天声,陈超森. 含氟卟啉的研究进展[J]. 有机化学, 2007, 27(01): 24-33. |
| [15] | 罗新湘,黄筱玲,曲凡歧. 几个新型含氟化合物的合成[J]. 有机化学, 2006, 26(06): 874-877. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||