有机化学 ›› 2022, Vol. 42 ›› Issue (11): 3747-3756.DOI: 10.6023/cjoc202206005 上一篇 下一篇
研究论文
孟祥辉, 杨亮茹*( ), 刘琪琳, 董振华, 袁金伟*(
), 刘琪琳, 董振华, 袁金伟*( ), 肖咏梅, 毛璞*(
), 肖咏梅, 毛璞*( )
)
收稿日期:2022-06-03
修回日期:2022-07-08
发布日期:2022-07-20
通讯作者:
杨亮茹, 袁金伟, 毛璞
基金资助:
Xianghui Meng, Liangru Yang( ), Qilin Liu, Zhenhua Dong, Jinwei Yuan(
), Qilin Liu, Zhenhua Dong, Jinwei Yuan( ), Yongmei Xiao, Pu Mao(
), Yongmei Xiao, Pu Mao( )
)
Received:2022-06-03
Revised:2022-07-08
Published:2022-07-20
Contact:
Liangru Yang, Jinwei Yuan, Pu Mao
Supported by:文章分享

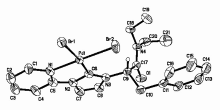
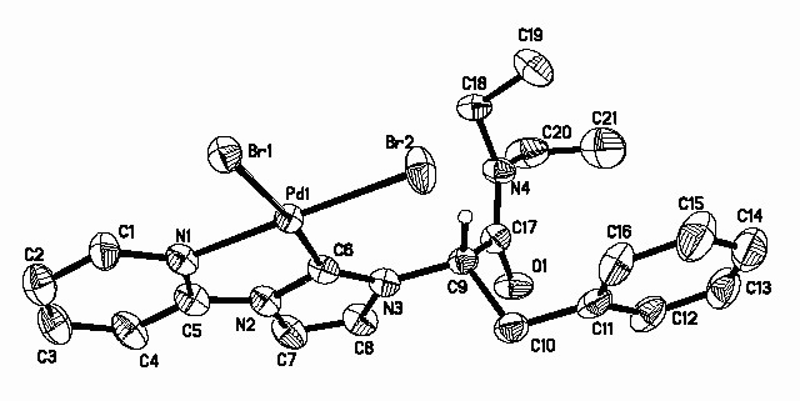
以氨基酸为原料, 合成了一系列N-烷基酰胺-N'-吡啶/嘧啶双官能团化的咪唑鎓盐, 室温下与Pd(OAc)2在CH2Cl2溶液中经直接金属化反应生成吡啶/嘧啶螯合的氮杂环卡宾钯化合物. 所得化合物的结构经核磁共振(NMR)、质谱(MS)或元素分析(EA)鉴定, 其中吡啶螯合氮杂环卡宾钯化合物5c的结构进一步经X射线单晶衍射确证. 催化活性研究表明, 使用二甲基乙酰胺(DMAc)为溶剂、KOAc为碱时, 这些化合物对咪唑衍生物的C-5芳基化反应表现出很高的催化活性.
孟祥辉, 杨亮茹, 刘琪琳, 董振华, 袁金伟, 肖咏梅, 毛璞. 酰胺功能化吡啶/嘧啶螯合氮杂环卡宾钯化合物的合成、结构及其催化的咪唑C-5芳基化反应[J]. 有机化学, 2022, 42(11): 3747-3756.
Xianghui Meng, Liangru Yang, Qilin Liu, Zhenhua Dong, Jinwei Yuan, Yongmei Xiao, Pu Mao. Amide Functionalized Pyridine/Pyrimidine Chelating N-Heterocyclic Carbene Palladium Complexes: Synthesis, Structure, and Catalysis for C-5 Arylation of Imidazoles[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3747-3756.

| Entrya | NHC-Pd (mol%) | Yieldb/% |
|---|---|---|
| 1 | 5a (2.0) | 65 |
| 2 | 5b (2.0) | 79 |
| 3 | 5c (2.0) | 74 |
| 4 | 5d (2.0) | 81 |
| 5 | 5e (2.0) | 76 |
| 6 | 5f (2.0) | 99 |
| 7 | 5f (1.0) | 87 |
| 8 | C1 (2.0) | 55 |
| 9 | C2 (2.0) | 67 |
| 10 | C3 (2.0) | 57 |
| 11 | C4 (2.0) | 89 |
| Entrya | NHC-Pd (mol%) | Yieldb/% |
|---|---|---|
| 1 | 5a (2.0) | 65 |
| 2 | 5b (2.0) | 79 |
| 3 | 5c (2.0) | 74 |
| 4 | 5d (2.0) | 81 |
| 5 | 5e (2.0) | 76 |
| 6 | 5f (2.0) | 99 |
| 7 | 5f (1.0) | 87 |
| 8 | C1 (2.0) | 55 |
| 9 | C2 (2.0) | 67 |
| 10 | C3 (2.0) | 57 |
| 11 | C4 (2.0) | 89 |
| Entrya | Solvent | Base | Temp./℃ | Yieldb/% |
|---|---|---|---|---|
| 1 | DMAc | KOAc | 140 | 99 |
| 2 | DMF | KOAc | 140 | 84 |
| 3 | DMSO | KOAc | 140 | 90 |
| 4 | Toluene | KOAc | 110 | 65 |
| 5 | 1,4-Dioxane | KOAc | 110 | 0 |
| 6 | THF | KOAc | 60 | 0 |
| 7 | DMAc | NaOAc | 140 | 34 |
| 8 | DMAc | K2CO3 | 140 | 61 |
| 9 | DMAc | K3PO4•3H2O | 140 | 55 |
| 10 | DMAc | K3PO4 | 140 | 68 |
| 11 | DMAc | Cs2CO3 | 140 | 0 |
| 12 | DMAc | NaH | 140 | 0 |
| Entrya | Solvent | Base | Temp./℃ | Yieldb/% |
|---|---|---|---|---|
| 1 | DMAc | KOAc | 140 | 99 |
| 2 | DMF | KOAc | 140 | 84 |
| 3 | DMSO | KOAc | 140 | 90 |
| 4 | Toluene | KOAc | 110 | 65 |
| 5 | 1,4-Dioxane | KOAc | 110 | 0 |
| 6 | THF | KOAc | 60 | 0 |
| 7 | DMAc | NaOAc | 140 | 34 |
| 8 | DMAc | K2CO3 | 140 | 61 |
| 9 | DMAc | K3PO4•3H2O | 140 | 55 |
| 10 | DMAc | K3PO4 | 140 | 68 |
| 11 | DMAc | Cs2CO3 | 140 | 0 |
| 12 | DMAc | NaH | 140 | 0 |
| Entry a | Imidazole | Bromide | Yieldb/% | |
|---|---|---|---|---|
| 1 | 1,2-Dimethylimidazole | p-BrC6H4COCH3 | 99 | |
| 2 | 1,2-Dimethylimidazole | m-BrC6H4COCH3 | 83 | |
| 3 | 1,2-Dimethylimidazole | o-BrC6H4COCH3 | 0 | |
| 4 | 1,2-Dimethylimidazole | p-BrC6H4CHO | 99 | |
| 5 | 1,2-Dimethylimidazole | m-BrC6H4CHO | 84 | |
| 6 | 1,2-Dimethylimidazole | o-BrC6H4CHO | 70 | |
| 7 | 1,2-Dimethylimidazole | p-BrC6H4CN | 96 | |
| 8 | 1,2-Dimethylimidazole | p-BrC6H4CH2CN | 74 | |
| 9 | 1,2-Dimethylimidazole | p-BrC6H4CF3 | 91 | |
| 10 | 1,2-Dimethylimidazole | p-BrC6H4F | 90 | |
| 11 | 1,2-Dimethylimidazole | C6H5Br | 66 | |
| 12 | 1,2-Dimethylimidazole | p-BrC6H4CH3 | 54 | |
| 13 | 1,2-Dimethylimidazole | m-BrC6H4CH3 | 51 | |
| 14 | 1,2-Dimethylimidazole | o-BrC6H4CH3 | 0 | |
| 15 | 1,2-Dimethylimidazole | p-BrC6H4OCH3 | 49 | |
| 16 | 1,2-Dimethylimidazole | m-BrC6H4OCH3 | 56 | |
| 17 | 1,2-Dimethylimidazole | o-BrC6H4OCH3 | 0 | |
| 18 | 1-Methylimidazole | p-BrC6H4COCH3 | 92 | |
| 19 | 1-Methylimidazole | p-BrC6H4CHO | 81 | |
| 21 | 1-Methylimidazole | m-BrC6H4CHO | 70 | |
| 22 | 1-Methylimidazole | o-BrC6H4CHO | 46 |
| Entry a | Imidazole | Bromide | Yieldb/% | |
|---|---|---|---|---|
| 1 | 1,2-Dimethylimidazole | p-BrC6H4COCH3 | 99 | |
| 2 | 1,2-Dimethylimidazole | m-BrC6H4COCH3 | 83 | |
| 3 | 1,2-Dimethylimidazole | o-BrC6H4COCH3 | 0 | |
| 4 | 1,2-Dimethylimidazole | p-BrC6H4CHO | 99 | |
| 5 | 1,2-Dimethylimidazole | m-BrC6H4CHO | 84 | |
| 6 | 1,2-Dimethylimidazole | o-BrC6H4CHO | 70 | |
| 7 | 1,2-Dimethylimidazole | p-BrC6H4CN | 96 | |
| 8 | 1,2-Dimethylimidazole | p-BrC6H4CH2CN | 74 | |
| 9 | 1,2-Dimethylimidazole | p-BrC6H4CF3 | 91 | |
| 10 | 1,2-Dimethylimidazole | p-BrC6H4F | 90 | |
| 11 | 1,2-Dimethylimidazole | C6H5Br | 66 | |
| 12 | 1,2-Dimethylimidazole | p-BrC6H4CH3 | 54 | |
| 13 | 1,2-Dimethylimidazole | m-BrC6H4CH3 | 51 | |
| 14 | 1,2-Dimethylimidazole | o-BrC6H4CH3 | 0 | |
| 15 | 1,2-Dimethylimidazole | p-BrC6H4OCH3 | 49 | |
| 16 | 1,2-Dimethylimidazole | m-BrC6H4OCH3 | 56 | |
| 17 | 1,2-Dimethylimidazole | o-BrC6H4OCH3 | 0 | |
| 18 | 1-Methylimidazole | p-BrC6H4COCH3 | 92 | |
| 19 | 1-Methylimidazole | p-BrC6H4CHO | 81 | |
| 21 | 1-Methylimidazole | m-BrC6H4CHO | 70 | |
| 22 | 1-Methylimidazole | o-BrC6H4CHO | 46 |
| [27] |
Sheldrick, G. M. Acta Cryst. 2015, C71, 3.
|
| [1] |
(a) Arduengo III, A. J.; Harlow, R. L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361.
doi: 10.1021/ja00001a054 |
|
(b) Arduengo III, A. J.; Kline, M.; Calabrese, J. C.; Davidson, F. J. Am. Chem. Soc. 1991, 113, 9704.
doi: 10.1021/ja00025a063 |
|
| [2] |
(a) Nolan, S. P. N-Heterocyclic Carbenes in Synthesis, Wiley-VCH, Weinheim, 2006.
pmid: 21731956 |
|
(b) Kühl, O. Chem. Soc. Rev. 2007, 36, 592.
doi: 10.1039/B603765H pmid: 21731956 |
|
|
(c) Fortman, G. C.; Nolan, S. P. Chem. Soc. Rev. 2011, 40, 5151.
doi: 10.1039/c1cs15088j pmid: 21731956 |
|
|
(d) Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. Nature 2014, 510, 485.
doi: 10.1038/nature13384 pmid: 21731956 |
|
|
(e) Chen, J.; Huang, Y. Nat. Commun. 2014, 5, 3437.
doi: 10.1038/ncomms4437 pmid: 21731956 |
|
|
(f) Yan, J.; Wang, Y. B.; Zhu, Z. H.; Li, Y.; Zhu, X.; Hao, X. Q.; Song, M. P. Organometallics 2018, 37, 2325.
doi: 10.1021/acs.organomet.8b00300 pmid: 21731956 |
|
|
(g) Peris. E. Chem. Rev. 2018, 118, 9988.
doi: 10.1021/acs.chemrev.6b00695 pmid: 21731956 |
|
|
(h) Thongpaen, J.; Manguin, R.; Baslé, O. Angew. Chem., Int. Ed. 2019, 59, 10242.
doi: 10.1002/anie.201911898 pmid: 21731956 |
|
| [3] |
(a) Bernhammer, J. C.; Huynh, H. V. Organometallics 2014, 33, 172.
doi: 10.1021/om400929t pmid: 28492647 |
|
(b) Yang, L.; Zhang, X.; Mao, P.; Xiao, Y.; Bian, H.; Yuan, J.; Mai, W.; Qu, L. RSC Adv. 2015, 5, 25723.
doi: 10.1039/C5RA01706H pmid: 28492647 |
|
|
(c) Liu, J.-Q.; Gou, X.-X.; Han, Y.-F. Chem. Asian J. 2018, 13, 2257.
doi: 10.1002/asia.201800583 pmid: 28492647 |
|
|
(d) Asensio, J. M.; Gómez-Sal, P.; Andrés, R.; de Jesús, E. Dalton Trans. 2017, 46, 6785.
doi: 10.1039/c7dt00643h pmid: 28492647 |
|
|
(e) Kaloğlu, N.; Kaloğlu, M.; Tahir, M. N.; Arıcı, C.; Bruneau, C.; Doucet, H.; Dixneuf, P. H.; Çetinkaya, B.; Özdemir, İ. J. Organomet. Chem. 2018, 867, 404.
doi: 10.1016/j.jorganchem.2017.10.019 pmid: 28492647 |
|
|
(f) Gardiner, M. G.; Ho, C. C. Coord. Chem. Rev. 2018, 375, 373.
doi: 10.1016/j.ccr.2018.02.003 pmid: 28492647 |
|
|
(g) An, Y.-Y.; Yu, J.-G.; Han, Y.-F. Chin. J. Chem. 2019, 37, 76.
doi: 10.1002/cjoc.201800450 pmid: 28492647 |
|
|
(h) Chen, W.; Yang, J. J. Organomet. Chem. 2018, 872, 24.
doi: 10.1016/j.jorganchem.2018.07.029 pmid: 28492647 |
|
|
(i) Bernhammer, J. C.; Huynh, H. V. Organometallics 2014, 33, 172.
doi: 10.1021/om400929t pmid: 28492647 |
|
| [4] |
(a) Peris, E.; Crabtree, R. H. Coord. Chem. Rev. 2004, 248, 2239.
doi: 10.1016/j.ccr.2004.04.014 pmid: 28083579 |
|
(b) Pugh, D.; Danopoulos, A. A. Coord. Chem. Rev. 2007, 251, 610.
doi: 10.1016/j.ccr.2006.08.001 pmid: 28083579 |
|
|
(c) Normand, A. T.; Cavell, K. J. Eur. J. Inorg. Chem. 2008, 2008, 2781.
doi: 10.1002/ejic.200800323 pmid: 28083579 |
|
|
(d) Hameury, S.; de Frémont, P.; Braunstein, P. Chem. Soc. Rev. 2017, 46, 632.
doi: 10.1039/c6cs00499g pmid: 28083579 |
|
|
(e) Fliedel, C.; Labande, A.; Manoury, E.; Poli, R. Coord. Chem. Rev. 2019, 394, 65.
doi: 10.1016/j.ccr.2019.05.003 pmid: 28083579 |
|
| [5] |
(a) Bhat, I. A.; Avinash, I.; Anantharaman, G. Organometallics 2019, 38, 1699.
doi: 10.1021/acs.organomet.8b00878 pmid: 34093085 |
|
(b) Lu, S. J.; Yang, H. H.; Chang, W. J.; Hsueh, H. H.; Lin, Y. C.; Liu, F. C.; Lin, I. J. B.; Lee, G. H. J. Organomet. Chem. 2020, 927, 121543.
doi: 10.1016/j.jorganchem.2020.121543 pmid: 34093085 |
|
|
(c) Bertini, S.; Albrecht, M. Chimia 2020, 74, 483.
doi: 10.2533/chimia.2020.483 pmid: 34093085 |
|
|
(d) Bertini, S.; Albrecht, M. Green Chem. 2021, 23, 3365.
doi: 10.1039/d1gc00388g pmid: 34093085 |
|
|
(e) La Cruz-Sánchez, P. de.; Faiges, J.; Mazloomi, Z.; Borràs, C.; Biosca, M.; Pàmies, O.; Diéguez, M. Organometallics 2019, 38, 4193.
doi: 10.1021/acs.organomet.9b00514 pmid: 34093085 |
|
|
(f) Bernier, C. M.; Merola, J. S. Catalysts 2021, 11, 671.
doi: 10.3390/catal11060671 pmid: 34093085 |
|
|
(g) Kwak, J.; Ohk, Y.; Jung, Y.; Chang, S. J. Am. Chem. Soc. 2012, 134, 17778.
doi: 10.1021/ja308205d pmid: 34093085 |
|
|
(h) Gandolfi, C.; Heckenroth, M.; Neels, A.; Laurenczy, G.; Albrecht, M. Organometallics 2009, 28, 5112.
doi: 10.1021/om900356w pmid: 34093085 |
|
|
(i) Endo, K.; Grubbs, R. H. J. Am. Chem. Soc. 2011, 133, 8525.
doi: 10.1021/ja202818v pmid: 34093085 |
|
| [6] |
Allolio, C.; Strassner, T. J. Org. Chem. 2014, 79, 12096.
doi: 10.1021/jo501897s |
| [7] |
Andrade, G. A.; DiMeglio, J. L.; Guardino, E. T.; Yap, G. P. A.; Rosenthal, J. Polyhedron 2017, 135, 34.
|
| [8] |
Sharma, K. N.; Satrawala, N.; Srivastava, A. K.; Ali, M.; Joshi, R. K. Org. Biomol. Chem. 2019, 17, 8969.
doi: 10.1039/c9ob01674k pmid: 31576395 |
| [9] |
Nayan Sharma, K.; Satrawala, N.; Kumar Joshi, R. Eur. J. Inorg. Chem. 2018, 1743.
|
| [10] |
(a) Bellina, F.; Cauteruccio, S.; Rossi, R. Tetrahedron 2007, 63, 4571.
doi: 10.1016/j.tet.2007.02.075 |
|
(b) Li, J. J.; Gribble, G. Palladium in Heterocyclic Chemistry, Pergamon, Amsterdam, Netherlands, 2000.
|
|
| [11] |
(a) Li, W.; Nelson, D. P.; Jensen, M. S.; Hoerrner, R. S.; Javadi, G. J.; Cai, D.; Larsen, R. D. Org. Lett. 2003, 5, 4835.
doi: 10.1021/ol035878k |
|
(b) Yoon, H.; Lossouarn, A.; Landau, F.; Lautens, M. Org. Lett. 2016, 18, 6324.
doi: 10.1021/acs.orglett.6b03213 |
|
|
(c) Chen, L.; Zhang, X.; Chen, B.; Li, B.; Li, Y. Chem. Heterocycl. Compd. 2017, 53, 618.
doi: 10.1007/s10593-017-2101-1 |
|
|
(d) Liu, L.; Liu, Y.; Ling, B.; Bi, S. J. Organomet. Chem. 2017, 827, 56.
doi: 10.1016/j.jorganchem.2016.11.001 |
|
| [12] |
(a) Ohno, H.; Iuchi, M.; Fujii, N.; Tanaka, T. Org. Lett. 2007, 9, 4813.
doi: 10.1021/ol702141r |
|
(b) Chang, S.-T.; Li, Q.; Chiang, R.-T.; Gau, H.-M. Tetrahedron 2012, 68, 3956.
doi: 10.1016/j.tet.2012.03.072 |
|
|
(c) Ma, X.; Liu, Y.; Liu, P.; Xie, J.; Dai, B.; Liu, Z. Appl. Organomet. Chem. 2014, 28, 180.
doi: 10.1002/aoc.3106 |
|
|
(d) Pei, K.; Jie, X.; Zhao, H.; Su, W. Eur. J. Org. Chem. 2014, 2014, 4230.
|
|
|
(e) Minami, Y.; Kodama, T.; Hiyama, T. Angew. Chem., Int. Ed. 2015, 54, 11813.
doi: 10.1002/anie.201505789 |
|
| [13] |
(a) Bellina, F.; Cauteruccio, S.; Di Fiore, A.; Marchetti, C.; Rossi, R. Tetrahedron 2008, 64, 6060.
doi: 10.1016/j.tet.2008.01.051 |
|
(b) Shibahara, F.; Yamauchi, T.; Yamaguchi, E.; Murai, T. J. Org. Chem. 2012, 77, 8815.
doi: 10.1021/jo301621t |
|
| [14] |
(a) Chiong, H. A.; Daugulis, O. Org. Lett. 2007, 9, 1449.
doi: 10.1021/ol0702324 |
|
(b) Joo, J. M.; Touré, B. B.; Sames, D. J. Org. Chem. 2010, 75, 4911.
doi: 10.1021/jo100727j |
|
|
(c) Powell, N. A.; Hagen, T. J.; Ciske, F. L.; Cai, C.; Duran, J. E.; Holsworth, D. D.; Leonard, D.; Kennedy, R M.; Edmunds, J. J. Tetrahedron Lett. 2010, 51, 4441.
doi: 10.1016/j.tetlet.2010.06.085 |
|
| [15] |
(a) Bensaid, S.; Laidaoui, N.; El Abed, D.; Kacimi, S.; Doucet, H. Tetrahedron Lett. 2011, 52, 1383.
doi: 10.1016/j.tetlet.2011.01.084 |
|
(b) Xu, X.; Zhao, L.; Li, Y.; Soulé, J.-F.; Doucet, H. Adv. Synth. Catal. 2015, 357, 2869.
doi: 10.1002/adsc.201500332 |
|
| [16] |
Ackermann, L.; Althammer, A.; Fenner, S. Angew. Chem., Int. Ed. 2009, 48, 201.
doi: 10.1002/anie.200804517 |
| [17] |
(a) Cacchi, S.; Fabrizi, G.; Goggiamani, A.; Licandro, E.; Maiorana, S.; Perdicchia, D. Org. Lett. 2005, 7, 1497.
doi: 10.1021/ol050130i |
|
(b) Ji, Y.; Plata, R. E.; Regens, C. S.; Hay, M.; Schmidt, M.; Razler, T. M.; Qiu, Y.; Geng, P.; Hsiao, Y.; Rosner, T.; Eastgate, M. D.; Blackmond, D. G. J. Am. Chem. Soc. 2015, 137, 13272.
doi: 10.1021/jacs.5b01913 |
|
|
(c) Ruch, A. A.; Handa, S.; Kong, F.; Nesterov, V. N.; Pahls, D. R.; Cundari, T. R.; Slaughter, L. M. Org. Biomol. Chem. 2016, 14, 8123.
doi: 10.1039/C6OB01102K |
|
| [18] |
Kumar, P. V.; Lin, W.-S.; Shen, J.-S.; Nandi, D.; Lee, H. M. Organometallics 2011, 30, 5160.
doi: 10.1021/om200490k |
| [19] |
(a) He, X. X.; Li, Y.; Ma, B. B.; Ke, Z.; Liu, F. S. Organometallics 2016, 35, 2655.
doi: 10.1021/acs.organomet.6b00391 |
|
(b) Hu, L. Q.; Deng, R. L.; Li, Y. F.; Zeng, C. J.; Shen, D. S.; Liu, F. S. Organometallics 2018, 37, 214.
doi: 10.1021/acs.organomet.7b00784 |
|
| [20] |
Li, H. H.; Maitra, R.; Kuo, Y. T.; Chen, J. H.; Hu, C. H.; Lee, H. M. Appl. Organomet. Chem. 2017, 32, e3956.
|
| [21] |
Bhaskar, R.; Sharma, A. K.; Singh, A. K. Organometallics 2018, 37, 2669.
doi: 10.1021/acs.organomet.8b00246 |
| [22] |
Bhatt, R.; Bhuvanesh, N.; Sharma, K. N.; Joshi, H. Eur. J. Inorg. Chem. 2020, 6, 532.
|
| [23] |
(a) Lee, J. Y.; Cheng, P. Y.; Tsai, Y. H.; Lin, G. R.; Liu, S. P.; Sie, M. H.; Lee, H. M. Organometallics 2010, 29, 3901.
doi: 10.1021/om1006402 |
|
(b) Kamisue, R.; Sakaguchi, S. J. Organomet. Chem. 2011, 696, 1910.
doi: 10.1016/j.jorganchem.2011.02.009 |
|
|
(c) Jahier-Diallo, C.; Morin, M. S. T.; Queval, P.; Rouen, M.; Artur, I.; Querard, P.; Toupet, L.; Crevisy, C.; Basle, O.; Mauduit, M. Chem. Eur. J. 2015, 21, 993.
doi: 10.1002/chem.201405765 |
|
| [24] |
(a) Yang, L.; Yuan, J.; Mao, P.; Guo, Q. RSC Adv. 2015, 5, 107601.
doi: 10.1039/C5RA21183B |
|
(b) Yang, L.; Zhang, W.; Xiao, Y.; Mao, P. ChemistrySelect 2016, 1, 680.
doi: 10.1002/slct.201600069 |
|
|
(c) Yang, L.; Zhang, X.; Yuan, J.; Xiao, Y.; Mao, P. J. Organomet. Chem. 2016, 818, 179.
doi: 10.1016/j.jorganchem.2016.06.015 |
|
|
(d) Yang, L.; Li, Y.; Mao, P.; Yuan, J.; Xiao, Y. Phosphorus, Sulfur Silicon Relat. Elem. 2019, 194, 780.
doi: 10.1080/10426507.2018.1546177 |
|
| [25] |
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Cryst. 2009, 42, 339.
doi: 10.1107/S0021889808042726 |
| [26] |
(a) Palatinus, L.; Chapuis, G. J. Appl. Cryst. 2007, 40, 786.
doi: 10.1107/S0021889807029238 |
|
(b) Palatinus, L.; van der Lee, A. J. Appl. Cryst. 2008, 41, 975.
doi: 10.1107/S0021889808028185 |
|
|
(c) Palatinus, L.; Prathapa, S. J.; van Smaalen, S. J. Appl. Cryst. 2012, 45, 575.
doi: 10.1107/S0021889812016068 |
| [1] | 夏登鹏, 罗锦昀, 何林, 蔡志华, 杜广芬. 氮杂环卡宾催化的五氟苯基硫醚的合成[J]. 有机化学, 2024, 44(2): 622-630. |
| [2] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [3] | 蔡远林, 吕亚, 聂桂花, 金智超, 池永贵. 氮杂环卡宾催化合成氰基化合物的研究进展[J]. 有机化学, 2023, 43(9): 3135-3145. |
| [4] | 杨亮茹, 郭梦丽, 袁金伟, 王佳美, 夏宇婷, 肖咏梅, 毛璞. 钳形氮杂环卡宾金属络合物的研究进展[J]. 有机化学, 2023, 43(6): 2002-2025. |
| [5] | 戴春波, 夏思奇, 陈晓玉, 杨丽敏. 氮杂环卡宾(NHC)催化[4+3]环加成反应构建4-氨基苯并环庚烯内酯[J]. 有机化学, 2023, 43(3): 1084-1090. |
| [6] | 刘婷婷, 胡宇才, 沈安. 亚胺配体协同氮杂环卡宾钯配合物催化碳碳偶联反应的作用机制[J]. 有机化学, 2023, 43(2): 622-628. |
| [7] | 赵薇, 刘京, 何向奎, 蒋豪, 陆良秋, 肖文精. 氮杂环卡宾(NHC)催化的联芳基二醛去对称化构建轴手性醛类化合物[J]. 有机化学, 2022, 42(8): 2504-2514. |
| [8] | 姚婷, 李佳燕, 王佳明, 赵常贵. 氮杂环卡宾催化构筑含七元环结构的研究进展[J]. 有机化学, 2022, 42(4): 925-944. |
| [9] | 成立, 王文蓉, 孙玉倩, 李团结, 于晨侠, 姚昌盛. 氮杂环卡宾(NHC)/银(I)共催化合成2-氧代-2-芳乙基芳甲酸酯[J]. 有机化学, 2021, 41(4): 1607-1613. |
| [10] | 张雨霞, 郭京程, 黄杰, 付振乾. 氮杂环卡宾催化乙酸酯和β-硅基烯酮的[4+2]环合反应:高立体选择性合成β-硅基δ-内酯[J]. 有机化学, 2021, 41(11): 4467-4475. |
| [11] | 张建明, 高健, 冯捷, 陆涛, 杜鼎. 氮杂环卡宾/过渡金属联合催化研究进展[J]. 有机化学, 2021, 41(10): 3792-3807. |
| [12] | 张阳, 邢芬, 冯泽男, 杜广芬, 顾承志, 何林. 氮杂环卡宾催化氰基乙酸酯与二烯酮双Michael反应:非对映选择性合成多取代环己酮和茚[J]. 有机化学, 2020, 40(6): 1608-1617. |
| [13] | 李莎, 徐嘉煜, 罗鲜, 杨雯涵, 姚昌盛. 氮杂环卡宾催化下官能化萘并吡喃酮的串联合成[J]. 有机化学, 2020, 40(2): 470-477. |
| [14] | 雍学锋, 黄建强, 何振宇. (NHC)Ni(II)催化的[3+2]氢烯化重排串联反应的应用[J]. 有机化学, 2020, 40(10): 3327-3337. |
| [15] | 王占林, 李如一, 钱辉旻, 姚昌盛. 温度对氮杂环卡宾催化下α-溴代烯醛与烯胺酮的[3+3]环化反应影响[J]. 有机化学, 2019, 39(7): 2075-2083. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||