有机化学 ›› 2023, Vol. 43 ›› Issue (7): 2368-2390.DOI: 10.6023/cjoc202211018 上一篇 下一篇
综述与进展
收稿日期:2022-11-16
修回日期:2023-02-09
发布日期:2023-03-06
通讯作者:
周俊
基金资助:
Fen Huang, Weiwei Luo, Jun Zhou( )
)
Received:2022-11-16
Revised:2023-02-09
Published:2023-03-06
Contact:
Jun Zhou
Supported by:文章分享
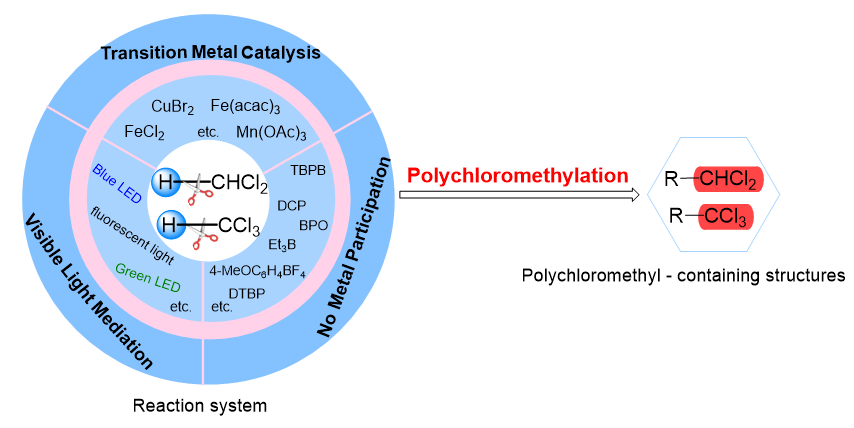
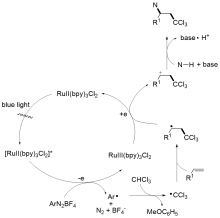
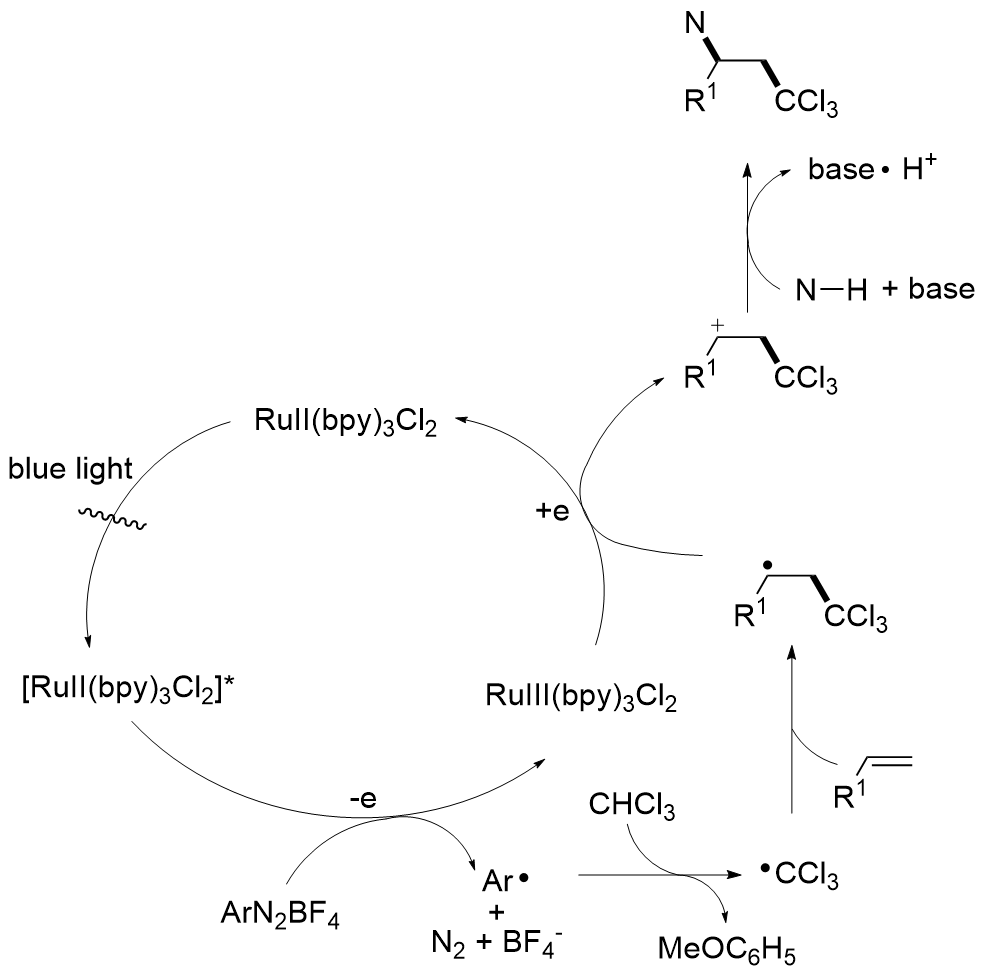
多氯代烃在有机合成和化学工业, 特别是制药和材料领域中尤其重要, 引起了化学家们的广泛关注, 诞生了各种合成氯代有机化合物的方法. 引入多氯烷基能够作为关键成分改变化合物的药物活性, 同时, 多氯烷基可以作为前体转化为醛、酮和羧酸等具有不同功能性的官能团, 为实现天然产物的后期合成和修饰提供强有力的支持. 通过断裂多氯烷烃中的C—H键来构建多氯代化合物的策略, 由于具有高步骤经济性, 已成为化学家们的研究热点, 取得了重要的研究进展. 总结了利用二氯甲烷、三氯甲烷作为多氯烷基的前体, 从不同的反应体系(过渡金属催化、可见光介导、无金属参与)出发, 综述了近十年来基于C—H键断裂策略的多氯烷基化反应的相关工作, 并对反应设计、机理研究、研究展望等给予评述.
黄芬, 罗维纬, 周俊. 基于C—H键断裂的多氯烷基化反应研究进展[J]. 有机化学, 2023, 43(7): 2368-2390.
Fen Huang, Weiwei Luo, Jun Zhou. Research Progress of Polychloroalkylation Based on C—H Bond Cleavage[J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2368-2390.
| [1] |
Jeschke, P. Pest Manage. Sci. 2010, 66, 10.
doi: 10.1002/ps.1829 |
| [2] |
Vaillancourt, F. H.; Yeh, E.; Vosburg, D. A.; Garneau-Tsodikova, S.; Walsh, C. T. Chem. Rev. 2006, 106, 3364.
pmid: 16895332 |
| [3] |
Unson, M. D.; Rose, C. B.; Faulkner, D. J.; Brinen, L. S.; Steiner, J. R.; Clardy, J. J. Org. Chem. 1993, 58, 6336.
doi: 10.1021/jo00075a029 |
| [4] |
Hughes, G.; Lewis, J. C. Chem. Rev. 2018, 118, 1.
doi: 10.1021/acs.chemrev.7b00741 pmid: 29316793 |
| [5] |
Sadar, M. D. Cancer Res. 2011, 71, 1208.
|
| [6] |
Gribble, G. W. Heterocycles 2012, 84, 157.
doi: 10.3987/REV-11-SR(P)5 |
| [7] |
Owusu-Ansah, E.; Durow, A. C.; Harding, J. R.; Jordan, A. C.; O’Connell, S. J.; Willis, C. L. Org. Biomol. Chem. 2011, 9, 265.
doi: 10.1039/c0ob00617c pmid: 21076771 |
| [8] |
Gribble, G. W. J. Chem. Educ. 2004, 81, 1441.
doi: 10.1021/ed081p1441 |
| [9] |
Gu, Z.; Zakarian, A. Angew. Chem., Int. Ed. 2010, 49, 9702.
doi: 10.1002/anie.201005354 |
| [10] |
Nguyen, V.-A.; Willis, C. L.; Gerwick, W. H. Chem. Commun. 2001, 19, 1934.
|
| [11] |
Flores, B.; Molinski, T. F. Org. Lett. 2011, 13, 3932.
doi: 10.1021/ol201461n |
| [12] |
Paul, C.; Pohnert, G. Nat. Prod. Rep. 2011, 28, 186.
doi: 10.1039/C0NP00043D |
| [13] |
Sadar, M. D.; Williams, D. E.; Mawji, N. R.; Patrick, B. O.; Wikanta, T.; Chasanah, E.; Irianto, H. E.; Soest, R. V.; Andersen, R. J. Org. Lett. 2008, 10, 4947.
doi: 10.1021/ol802021w |
| [14] |
Orjala, J.; Gerwick, W. H. J. Nat. Prod. 1996, 59, 427.
pmid: 8699186 |
| [15] |
Xiao, Q.; Young, K.; Zakarian, A. Org. Lett. 2013, 15, 3314.
doi: 10.1021/ol401354a |
| [16] |
Ardá, A.; Soengas, R. G.; Nieto, M. I.; Jiménez, C.; Rodríguez, J. Org. Lett. 2008, 10, 2175.
doi: 10.1021/ol800551g pmid: 18459798 |
| [17] |
Durow, A. C.; Long, G. C.; O'Connel, S. J.; Willis, C. L. Org. Lett. 2006, 8, 5401.
doi: 10.1021/ol062279f |
| [18] |
Fu, X.; Su, J.-Y.; Zeng, L.-M. Chin. J. Chem. 2000, 18, 882.
doi: 10.1002/cjoc.20000180616 |
| [19] |
Kapojos, M. M.; Abdjul, D. B.; Yamazaki, H.; Ohshiro, T.; Rotinsulu, H.; Wewengkang, D. S.; Sumilat, D. A.; Tomoda, H.; Namikoshi, M.; Uchida, R. Bioorg. Med. Chem. Lett. 2018, 28, 1911.
doi: S0960-894X(18)30283-X pmid: 29631961 |
| [20] |
Zada, S. L.; Green, K. D.; Shrestha, S. K.; Herzog, I. M.; Garneau- Tsodikova, S.; Fridman, M. ACS Infect. Dis. 2018, 4, 1121.
doi: 10.1021/acsinfecdis.8b00078 |
| [21] |
Bao, Y.; Wang, G.-Y.; Zhang, Y.-X.; Bian, K.-J.; Wang, X.-S. Chem. Sci. 2018, 9, 2986.
doi: 10.1039/C8SC00210J |
| [22] |
Shimakoshi, H.; Luo, Z.; Inaba, T.; Hisaeda, Y. Dalton Trans. 2016, 45, 10173.
doi: 10.1039/c6dt00556j pmid: 27071703 |
| [23] |
Sharma, P.; Rohilla, S.; Jain, N. J. Org. Chem. 2017, 82, 1105.
doi: 10.1021/acs.joc.6b02711 |
| [24] |
Liu, X.; Li, B.; Gu, Z. J. Org. Chem. 2015, 80, 7547.
doi: 10.1021/acs.joc.5b01126 |
| [25] |
Brennführer, A.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2009, 48, 4114.
doi: 10.1002/anie.200900013 pmid: 19431166 |
| [26] |
Luo, W.; Jiang, K.; Li, Y.; Jiang, H.; Yin, B. Org. Lett. 2020, 22, 2093.
doi: 10.1021/acs.orglett.0c00582 |
| [27] |
Kharasch, M. S.; Jensen, E. V.; Urry, W. H. Science 1945, 102, 128.
pmid: 17777366 |
| [28] |
Vaillancourt, F. H.; Yeh, E.; Vosburg, D. A.; O’Connor, S. E.; Walsh, C. T. Nature 2005, 436, 1191.
doi: 10.1038/nature03797 |
| [29] |
Liang, Y.-Y.; Lv, G.-F.; Ouyang, X.-H.; Song, R.-J.; Li, J.-H. Adv. Synth. Catal. 2021, 363, 290.
doi: 10.1002/adsc.v363.2 |
| [30] |
Huang, G.; Yu, J.-T.; Pan, C.-D. Adv. Synth. Catal. 2021, 363, 305.
doi: 10.1002/adsc.v363.2 |
| [31] |
Liu, S.; Su, Y.-L.; Sun, T.-Y.; Doyle, M.P.; Wu, Y.-D.; Zhang, X. J. Am. Chem. Soc. 2021, 143, 13195.
doi: 10.1021/jacs.1c05208 |
| [32] |
Lu, M.-Z.; Loh, T.-P. Org. Lett. 2014, 16, 4698.
doi: 10.1021/ol502411c |
| [33] |
Chan, C.-W.; Lee, P.-Y.; Yu, W.-Y. Terahedron Lett. 2015, 56, 2559.
doi: 10.1016/j.tetlet.2015.03.109 |
| [34] |
Li, X.; Xu, J.; Gao, Y.; Fang, H.; Tang, G.; Zhao, Y. J. Org. Chem. 2015, 80, 2621.
doi: 10.1021/jo502777b |
| [35] |
Li, W.-Y.; Wu, C.-S.; Wang, Z.; Yang, L. Chem. Commun. 2018, 54, 11013.
doi: 10.1039/C8CC05090B |
| [36] |
Xu, L.; Chen, J.; Chu, L. Org. Chem. Front. 2019, 6, 512.
doi: 10.1039/C8QO01142G |
| [37] |
Liang, Y.-Y.; Huang, J.; Ouyang, X.-H.; Qin, J.-H.; Song, R.-J.; Li, J.-H. Chem. Commun. 2021, 57, 3684.
doi: 10.1039/D1CC00400J |
| [38] |
Zhao, Z.-W.; Ran, Y.-S.; Hou, Y.-J.; Chen, X.; Ding, X.-L.; Zhang, C.; Li, Y.-M. J. Org. Chem. 2022, 87, 4183.
doi: 10.1021/acs.joc.1c03024 |
| [39] |
Zhang, J.-H.; Jiang, L.-L.; Hu, S.-J.; Li, J.-Z.; Yu, X.-C.; Liu, F.-L.; Guan, Y.-T.; Lei, K.-W.; Wei, W.-T. Org. Biomol. Chem. 2022, 20, 7067.
doi: 10.1039/D2OB01330D |
| [40] |
Luo, W.; Jiang, K.; Yin, B. Chin. J. Chem. 2022, 40, 2893.
doi: 10.1002/cjoc.v40.24 |
| [41] |
Liu, Y.; Zhang, J.-L.; Song, R.-J.; Li, J.-H. Eur. J. Org. Chem. 2014, 2014, 1177.
doi: 10.1002/ejoc.v2014.6 |
| [42] |
Liu, Y.; Zhang, J.-L.; Song, R.-J.; Li, J.-H. Org. Chem. Front. 2014, 1, 1289.
doi: 10.1039/C4QO00251B |
| [43] |
Liu, Y.; Song, R.-J.; Luo, S.; Li, J.-H. Org. Lett. 2018, 20, 212.
doi: 10.1021/acs.orglett.7b03561 pmid: 29261325 |
| [44] |
Huang, J.; Liang, Y.-Y.; Ouyang, X.-H.; Xiao, Y.-T.; Qin, J.-H.; Song, R.-J.; Li, J.-H. Org. Chem. Front. 2021, 8, 7009.
doi: 10.1039/D1QO01263K |
| [45] |
Wang, T.-L.; Zhang, B.-S.; Liu, J.-J.; Liu, X.-J.; Wang, X.-C.; Quan, Z.-J. Org. Chem. Front. 2022, 9, 1004.
doi: 10.1039/D1QO01662H |
| [46] |
Ma, N.; Guo, L.; Shen, Z.-J.; Qi, D.; Yang, C.; Xia, W. Org. Biomol. Chem. 2022, 20, 1731.
doi: 10.1039/D1OB02480A |
| [47] |
Behr, J.-B.; Chavaria, D.; Plantier-Royon, R. J. Org. Chem. 2013, 78, 11477.
doi: 10.1021/jo402028r |
| [48] |
Tian, Y.; Liu, Z.-Q. RSC Adv. 2014, 4, 64855.
doi: 10.1039/C4RA12032A |
| [49] |
Ueda, M.; Doi, N.; Miyagawa, H.; Sugita, S.; Takeda, N.; Shinada, T.; Miyata, O. Chem. Commun. 2015, 51, 4204.
doi: 10.1039/C4CC09649E |
| [50] |
Sheng, W.-J.; Jin, C.-A.; Shan, S.; Jia, Y.-X.; Gao, J.-R. Chin. J. Org. Chem. 2016, 36, 325 (in Chinese).
doi: 10.6023/cjoc201509008 |
|
(盛卫坚, 金城安, 单尚, 贾义霞, 高建荣, 有机化学, 2016, 36, 325.)
doi: 10.6023/cjoc201509008 |
|
| [51] |
Xu, J.-K.; Li, S.-J.; Wang, H.-Y.; Xu, W.-C.; Tian, S.-K. Chem. Commun. 2017, 53, 1708.
doi: 10.1039/C6CC09311F |
| [52] |
Pan, C.; Gao, D.; Yang, Z.; Wu, C.; Yu, J.-T. Org. Biomol. Chem. 2018, 16, 5752.
doi: 10.1039/C8OB01554F |
| [53] |
Li, W.-L.; Sun, Y.-T.; Yao, Y.-C.; Xu, Y.; Li, P.; Liu, Y.-J.; Liang, D.-Q. Chin. J. Org. Chem. 2019, 39, 1727 (in Chinese).
doi: 10.6023/cjoc201901047 |
|
(李文兰, 孙一茼, 姚永超, 许颖, 李鹏, 刘颖杰, 梁德强, 有机化学, 2019, 39, 1727.)
doi: 10.6023/cjoc201901047 |
|
| [54] |
Pan, C.; Wu, C.; Yuan, C.; Yu, J.-T. Tetrahedron Lett. 2020, 61, 151499.
doi: 10.1016/j.tetlet.2019.151499 |
| [55] |
Sun, M.; Xu, Z.; Li, W.; Yang, L.; You, H.; Yu, D.; Fang, F.; Wang, Y.; Liu, Z.-Q. Adv. Synth. Catal. 2020, 362, 2195.
doi: 10.1002/adsc.v362.11 |
| [56] |
Ge, Y.-X.; Yan, Q.-Q.; Tian, Y.-F.; Wang, H.-J.; Zhang, C.-F.; Li, Z.-J. Chin. J. Org. Chem. 2021, 41, 3106 (in Chinese).
doi: 10.6023/cjoc202102035 |
|
(葛雅欣, 闫芹芹, 田云飞, 王海军, 张春芳, 李泽江, 有机化学, 2021, 41, 3106.)
doi: 10.6023/cjoc202102035 |
|
| [57] |
Ren, Y.; Ge, Y.; Yan, Q.; Chen, S.; Li, Y.; Li, L.; Liu, Z.-Q.; Li, Z.-J. J. Org. Chem. 2021, 86, 12460.
doi: 10.1021/acs.joc.1c01605 |
| [58] |
Peng, C.-C.; Long, F.; Hu, Y.-C.; Zhou, Z.-R.; Zhang, K.-Y.; Wang, R.; Ye, M.-H.; Xiao, H.-B.; Wu, L.-J. J. Org. Chem. 2022, 87, 2740.
doi: 10.1021/acs.joc.1c02664 |
| [59] |
Liu, H.; Yang, Z.; Yu, J.-T.; Pan, C. Adv. Synth. Catal. 2022, 364, 1085.
doi: 10.1002/adsc.v364.6 |
| [60] |
Shan, Y.; Yang, Z.; Yu, J.-T.; Pan, C. Org. Biomol. Chem. 2022, 20, 5259.
doi: 10.1039/D2OB00471B |
| [61] |
Li, J.-N.; Li, Z.-J.; Shen, L.-Y.; Li, P.; Zhang, Y.; Yang, W.-C. Org. Biomol. Chem. 2022, 20, 6659.
doi: 10.1039/D2OB01053D |
| [1] | 田钰, 张娟, 高文超, 常宏宏. 二甲亚砜作为甲基化试剂在有机合成中的应用[J]. 有机化学, 2023, 43(7): 2391-2406. |
| [2] | 赵金晓, 魏彤辉, 柯森, 李毅. 可见光催化合成二氟烷基取代的多环吲哚化合物[J]. 有机化学, 2023, 43(3): 1102-1114. |
| [3] | 胡朝明, 吴纪红, 吴晶晶, 吴范宏. 直接三氟甲硒基化反应研究进展[J]. 有机化学, 2023, 43(1): 36-56. |
| [4] | 宇世伟, 陈兆华, 陈淇, 林舒婷, 何金萍, 陶冠燊, 汪朝阳. 硫代磺酸酯的合成与应用研究进展[J]. 有机化学, 2022, 42(8): 2322-2330. |
| [5] | 张力之, 廖永剑, 陈宁, 黄磊, 周敏. 叔丁醇钾促进的环化和偶联反应[J]. 有机化学, 2022, 42(7): 1950-1959. |
| [6] | 李亚东, 吴鹏举, 杨志勇. 可见光催化苯并噁唑与α-酮酸合成芳基苯并噁唑[J]. 有机化学, 2022, 42(6): 1770-1777. |
| [7] | 乐柏佟, 吴新鑫, 朱晨. 烯基自由基参与的分子内氢原子转移反应的新进展[J]. 有机化学, 2022, 42(2): 458-470. |
| [8] | 孙亚敏, 李锡勇, 袁金伟, 余加琳, 刘帅楠. 温和条件下以芳基胺为原料CuI催化下区域选择性合成3-芳基香豆素[J]. 有机化学, 2022, 42(2): 631-640. |
| [9] | 徐浩, 张杰, 左峻泽, 王丰晓, 吕健, 混旭, 杨道山. 硫鎓盐在可见光催化构建C—C键及C—杂原子键中的应用进展[J]. 有机化学, 2022, 42(12): 4037-4059. |
| [10] | 肖潜, 佟庆笑, 钟建基. 基于自由基串联环化反应合成苯并吖庚因衍生物的研究进展[J]. 有机化学, 2022, 42(12): 3979-3994. |
| [11] | 高盼盼, 肖文精, 陈加荣. 可见光促进的烯烃合成研究进展[J]. 有机化学, 2022, 42(12): 3923-3943. |
| [12] | 张孟琪, 南光明, 赵晓辉, 魏伟. 可见光介导喹喔啉-2(1H)-酮C3-H缩醛化反应[J]. 有机化学, 2022, 42(12): 4315-4322. |
| [13] | 许耀辉, 吴镇, 吴新鑫, 朱晨. 无过渡金属参与的醚、醛和酰胺C—H键自由基炔基化和烯丙基化反应[J]. 有机化学, 2022, 42(12): 4340-4349. |
| [14] | 吴业春, 于金涛. α-酮酸的脱羧酰基化/环化反应研究进展[J]. 有机化学, 2022, 42(11): 3606-3619. |
| [15] | 钱宇亮, 许岚, 童义涵, 刘天怿, 夏盈盈, 胡顶茂, 荣良策. 乙腈(丙酮)α-C嵌入合成2-取代环戊烯并[c]色烯衍生物[J]. 有机化学, 2022, 42(1): 181-189. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||