有机化学 ›› 2022, Vol. 42 ›› Issue (11): 3606-3619.DOI: 10.6023/cjoc202205025 上一篇 下一篇
综述与进展
收稿日期:2022-05-17
修回日期:2022-06-23
发布日期:2022-07-05
通讯作者:
于金涛
基金资助:Received:2022-05-17
Revised:2022-06-23
Published:2022-07-05
Contact:
Jintao Yu
Supported by:文章分享

α-酮酸的脱羧反应是得到酰基自由基的简便高效的方法, 而连续的自由基环化反应是合成环状结构, 特别是杂环结构的重要途径. 通过α-酮酸脱羧产生酰基自由基引发的自由基环化反应, 可以高效构建酰基化的吲哚酮、喹啉酮、菲啶、香豆素、色酮、苯并噻吩、吡咯[1,2-a]吲哚等结构. 介绍了α-酮酸脱羧产生酰基自由基而引发的自由基历程的酰基化/环化反应的最新进展.
吴业春, 于金涛. α-酮酸的脱羧酰基化/环化反应研究进展[J]. 有机化学, 2022, 42(11): 3606-3619.
Yechun Wu, Jintao Yu. Recent Advances in the Decarboxylative Acylation/Cyclization of α-Keto Acids[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3606-3619.



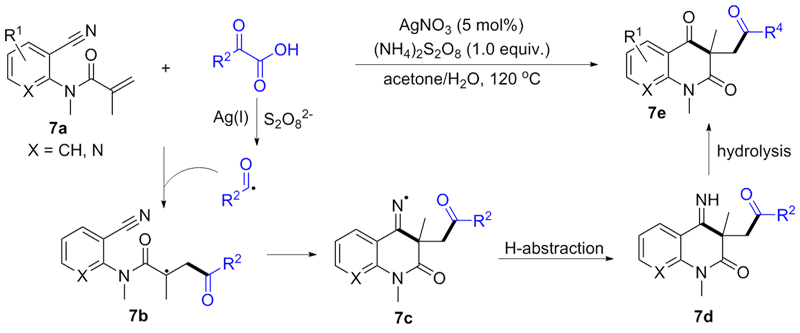

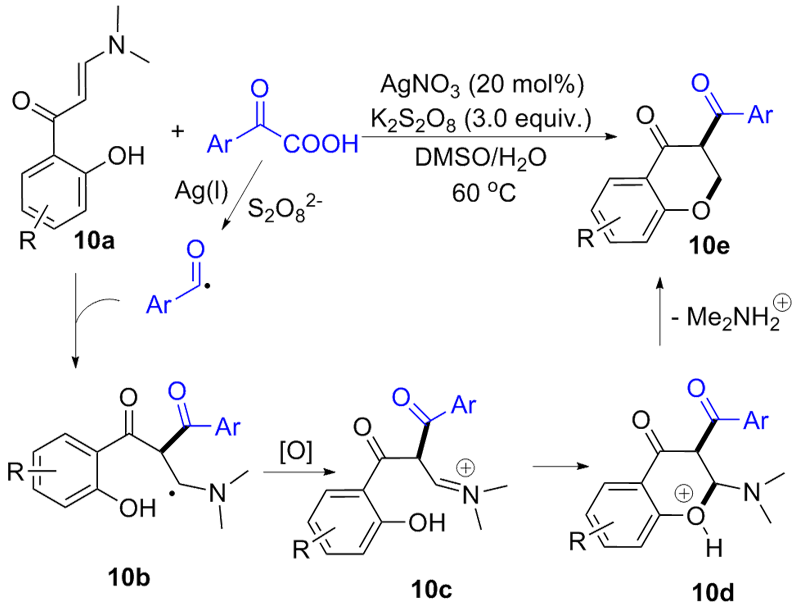

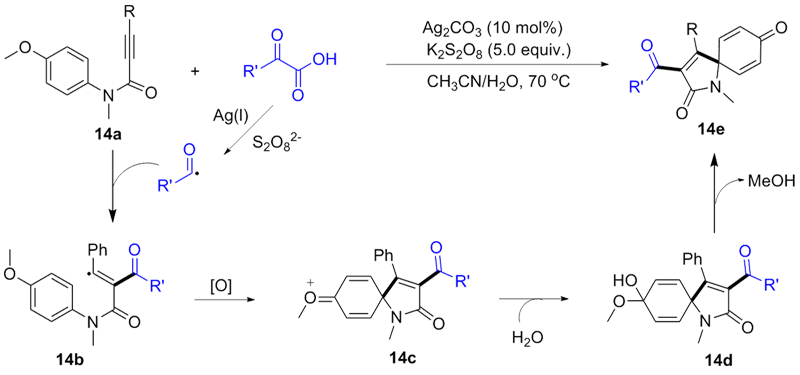

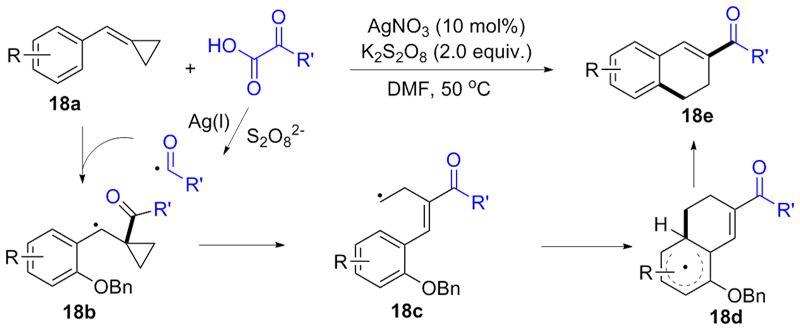







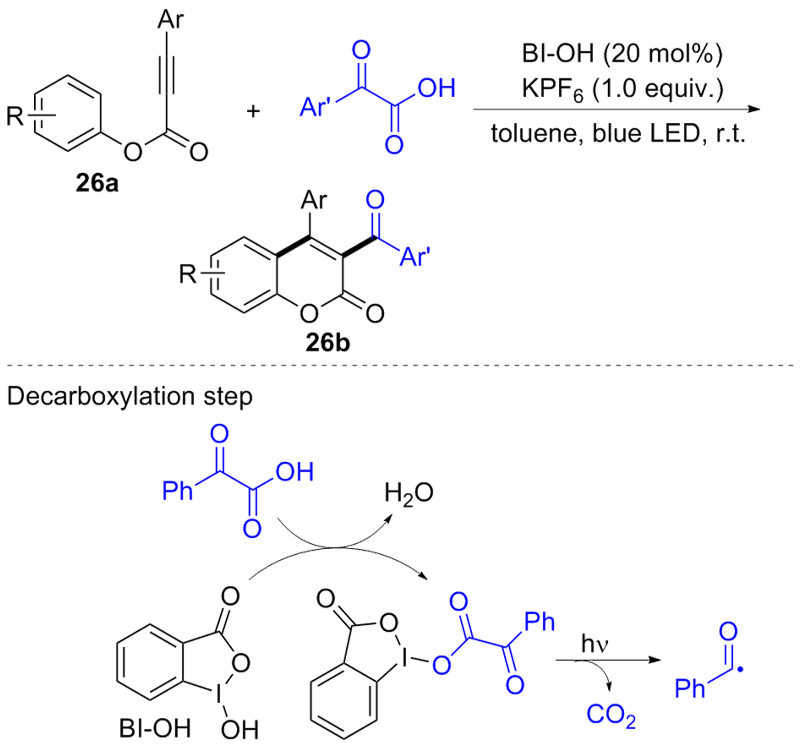
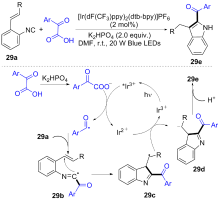





| [1] |
Kay, J.; Weitzman, P. D. J. Biochemical Society Symposium, Vol. 54, the Biochemical Society, London, 1987.
|
| [2] |
Fontana, F.; Minisci, F.; Barbosa, M. C. N.; Vismara, E. J. Org. Chem. 1991, 56, 2866.
doi: 10.1021/jo00008a050 |
| [3] |
(a) Rudolphi, L. J. F.; Oppel, C.; RodrIguez, N. Angew. Chem., nt. Ed. 2008, 47, 3043.
|
|
(b) Gooßen, L. J.; Zimmermann, B.; Knauber, T. Angew. Chem., nt. Ed. 2008, 47, 7103.
|
|
| [4] |
(a) Coppa, F.; Fontana, F.; Lazzarini, E.; Minisci, F. Heterocycles 1993, 36, 2687.
doi: 10.3987/COM-93-6459 pmid: 26061400 |
|
(b) Minisci, F.; Fontana, F.; Coppa, F.; Yan, Y. M. J. Org. Chem. 1995, 60, 5430.
doi: 10.1021/jo00122a020 pmid: 26061400 |
|
|
(c) Li, M.; Wang, C.; Fang, P.; Ge, H. Chem. Commun. 2011, 47, 6587.
doi: 10.1039/c1cc11635e pmid: 26061400 |
|
|
(d) Wang, H.; Guo, L. N.; Wang, S.; Duan, X.-H. Org. Lett. 2015, 17, 3054.
doi: 10.1021/acs.orglett.5b01336 pmid: 26061400 |
|
| [5] |
For reviews, see: (a) Li, Z.-L.; Fang, G.-C.; Gu, Q.-S.; Liu, X.-Y. Chem. Soc. Rev. 2020, 49, 32.
doi: 10.1039/C9CS00681H pmid: 32055811 |
|
(b) Li, Y.; Wu, D.; Cheng, H.; Yin, G. Angew. Chem., nt. Ed. 2020, 59, 7990.
pmid: 32055811 |
|
|
(c) Qi, X.; Diao, T. ACS Catal. 2020, 10, 8542.
doi: 10.1021/acscatal.0c02115 pmid: 32055811 |
|
|
(d) Jiang, H.; Studer, A. Chem. Soc. Rev. 2020, 49, 1790.
doi: 10.1039/c9cs00692c pmid: 32055811 |
|
|
(e) Yu, J.-T.; Pan, C. Chem. Commun. 2016, 52, 2220.
doi: 10.1039/C5CC08872K pmid: 32055811 |
|
|
(f) Liu, H.; Yu, J.-T.; Pan, C. Chem. Commun. 2021, 57, 6707.
doi: 10.1039/D1CC02322E pmid: 32055811 |
|
|
(g) Wang, W.; Zhang, M.; Yang, W.; Yang, X. Chin. J. Org. Chem. 2022, 42, 75. (in Chinese)
doi: 10.6023/cjoc202107012 pmid: 32055811 |
|
|
( 王弯弯, 张明明, 杨文超, 杨小虎, 有机化学 2022, 42, 75.)
doi: 10.6023/cjoc202107012 pmid: 32055811 |
|
| [6] |
Guo, L.-N.; Wang, H.; Duan, X.-H. Org. Biomol. Chem. 2016, 14, 7380.
doi: 10.1039/C6OB01113F |
| [7] |
Penteado, F.; Lopes, E. F..; Alves, D.; Perin, G.; Jacob, R. G.; Lenardão, E. J. Chem. Rev. 2019, 119, 7113.
|
| [8] |
Miao, J.; Ge, H. Synlett 2014, 25, 911.
doi: 10.1055/s-0033-1340174 |
| [9] |
For reviews, see: (a) Wolfe, J. P. Eur. J. Org. Chem. 2007, 571.
|
|
(b) Wolfe, J. P. Synlett 2008, 2913.
|
|
|
(c) Schultz, D. M.; Wolfe, J. P. Synthesis 2012, 351.
|
|
|
(d) Wolfe, J. P. Top. Heterocycl. Chem. 2013, 32, 1.
|
|
| [10] |
Wang, H.; Guo, L.-N.; Duan, X.-H. Adv. Synth. Catal. 2013, 355, 2222.
doi: 10.1002/adsc.201300468 |
| [11] |
Yang, H.; Guo, L.-N. and Guo, X.-H. RSC Adv. 2014, 4, 52986.
doi: 10.1039/C4RA08529A |
| [12] |
Mai, W.-P.; Sun, G.-C.; Wang, J.-T.; Song, G.; Mao, P.; Yang, L.-R.; Yuan, J.-W.; Xiao, Y.-M.; Qu, L.-B. J. Org. Chem. 2014, 79, 8094.
doi: 10.1021/jo501301t |
| [13] |
Liu, J.; Fan, C.; Yin, H.; Qin, C.; Zhang, G.; Zhang, X.; Yia, H.; Lei, A. Chem. Commun. 2014, 50, 2145.
doi: 10.1039/C3CC49026B |
| [14] |
Yan, K.; Yang, D.; Wei, W.; Wang, F.; Shuai, Y.; Li, Q.; Wang, H. J. Org. Chem. 2015, 80, 1550.
doi: 10.1021/jo502474z |
| [15] |
Liu, T.; Ding, Q.; Zong, Q.; Qiu, G. Org. Chem. Front. 2015, 2, 670.
doi: 10.1039/C5QO00029G |
| [16] |
Wang, S.-S.; Fu, H.; Shen, Y.; Sun, M.; Li, Y.-M. J. Org. Chem. 2016, 81, 2920.
doi: 10.1021/acs.joc.6b00210 |
| [17] |
Yang, W.-C.; Dai, P.; Luo, K.; Ji, Y.-G.; Wu, L. Adv. Synth. Catal. 2017, 359, 2390.
doi: 10.1002/adsc.201601407 |
| [18] |
Zhu, W.; Hu, Y.-Q.; Hong, X.-Y.; Li, G.-X.; Huang, X.-B.; Gao, W.-X.; Liu, M.-C.; Xia, Y.; Zhou, Y.-B.; Wu, H.-Y. Chem. Commun. 2018, 54, 14148.
doi: 10.1039/C8CC07735E |
| [19] |
Mkrtchyan, S.; Iaroshenko, V. O. Eur. J. Org. Chem. 2018, 6867.
|
| [20] |
Shang, J.-Q.; Wang, X.-X.; Xin, Y.; Li, Y.; Zhou, B.; Li, Y.-M. Org. Biomol. Chem. 2019, 17, 9447.
doi: 10.1039/C9OB02023C |
| [21] |
Chen, G.; Li, C.; Peng, J.; Yuan, Z.; Liu, P.; Liu, X. Org. Biomol. Chem. 2019, 17, 8527.
doi: 10.1039/C9OB01236B |
| [22] |
Jin, C.; He, J.-Y.; Bai, Q.-F.; Feng, G. Synlett 2020, 31, 1517.
doi: 10.1055/s-0040-1707891 |
| [23] |
Reddy, C. R.; Kolgave, D. H.; Subbarao, M.; Aila, M.; Prajapti, S. K. Org. Lett. 2020, 22, 5342.
doi: 10.1021/acs.orglett.0c01588 |
| [24] |
Reddy, C. R.; Kajare, R. C.; Punna, N. Chem. Commun. 2020, 56, 3445.
doi: 10.1039/C9CC10069E |
| [25] |
Zhang, Z.; Jia, C.; Kong, X.; Hussain, M.; Liu, Z.; Liang, W.; Jiang, L.; Jiang, H.; Ma, J. ACS Sustainable Chem. Eng. 2020, 8, 16463.
doi: 10.1021/acssuschemeng.0c05118 |
| [26] |
Gao, Q.; Jing, Q.; Chen, Y.; Sun, J.; Zhou, M. Chin. J. Org. Chem. 2022, 42, 257. (in Chinese)
doi: 10.6023/cjoc202105025 |
|
( 高启升, 荆祺, 陈阳, 孙京, 周明东, 有机化学 2022, 42, 257.)
doi: 10.6023/cjoc202105025 |
|
| [27] |
Zhang, J.; Wu, M.; Ju, H.; Yang, H.; Qian, B.; Ding, K.; Wu, J.; Xie, M. Org. Chem. Front. 2022, 9, 32.
doi: 10.1039/D1QO01069G |
| [28] |
(a) Rubin, M.; Rubina, M.; Gevorgyan, V. Chem. Rev. 2007, 107, 3117.
doi: 10.1021/cr050988l |
|
(b) Shao, L.-X.; Shi, M. Curr. Org. Chem. 2007, 11, 1135.
doi: 10.2174/138527207781662483 |
|
|
(c) Shi, M.; Shao, L.-X.; Lu, J.-M.; Wei, Y.; Mizuno, K.; Maeda, H. Chem. Rev. 2010, 110, 5883.
doi: 10.1021/cr900381k |
|
|
(d) Shi, M.; Lu, J.-M.; Wei, Y.; Shao, L.-X. Acc. Chem. Res. 2012, 45, 641.
doi: 10.1021/ar200237z |
|
|
(e) Brandi, A.; Cicchi, S.; Cordero, F. M.; Goti, A. Chem. Rev. 2014, 114, 7317.
doi: 10.1021/cr400686j |
|
|
(f) Zhang, D.-H.; Tang, X.-Y.; Shi, M. Acc. Chem. Res. 2014, 47, 913.
doi: 10.1021/ar400159r |
|
| [29] |
Liu, Y.; Chen, Z.; Wang, Q.-L.; Zhou, C.-S.; Xiong, B.-Q.; Yang, C.-A.; Tang, K.-W. J. Org. Chem. 2019, 84, 9984.
doi: 10.1021/acs.joc.9b01125 pmid: 31319024 |
| [30] |
Jing, K.; Cui, P.-C.; Wang, G.-W. Chem. Commun. 2019, 55, 12551.
doi: 10.1039/C9CC06460E |
| [31] |
(a) Mandal, S.; Bera, T.; Dubey, G.; Saha, J.; Laha, J. K. ACS Catal. 2018, 8, 5085.
doi: 10.1021/acscatal.8b00743 pmid: 26061400 |
|
(b) Wang, H.; Guo, L.-N.; Wang, S.; Duan, X.-H. Org. Lett. 2015, 17, 3054.
doi: 10.1021/acs.orglett.5b01336 pmid: 26061400 |
|
|
(c) Laha, J. K.; Patel, K. V. Chem. Commun. 2016, 52, 10245.
doi: 10.1039/C6CC04259G pmid: 26061400 |
|
|
(d) Laha, J. K.; Hunjan, M. K.; Hegde, S.; Gupta, A. Org. Lett. 2020, 22, 1442.
doi: 10.1021/acs.orglett.0c00041 pmid: 26061400 |
|
| [32] |
Yuan, M.; Chen, L.; Wang, J.; Chen, S.; Wang, K.; Xue, Y.; Yao, G.; Luo, Z.; Zhang, Y. Org. Lett. 2015, 17, 346.
doi: 10.1021/ol503459s |
| [33] |
Laha, J. K.; Patel, K. V.; Dubey, G.; Jethava, K. P. Org. Biomol. Chem. 2017, 15, 2199.
doi: 10.1039/C7OB00077D |
| [34] |
Nair, A. M.; Shinde, A. H.; Kumar, S.; Volla, C. M. R. Chem. Commun. 2020, 56, 12367.
doi: 10.1039/D0CC04800C |
| [35] |
Hu, X.-Y. ; Xu, H.-F. ; Chen, Q. Pan, Y.-L.; Chen, J.-Z. Org. Biomol. Chem. 2021, 19, 10376.
doi: 10.1039/d1ob01917a pmid: 34812822 |
| [36] |
Liu, Q.; Wang, L.; Liu, J.; Ruana, S.; Li, P. Org. Biomol. Chem. 2021, 19, 3489.
doi: 10.1039/d1ob00101a pmid: 33899870 |
| [37] |
Han, Q.-Q.; Sun, Y.-Y.; Yang, S.-H.; Song, J.-C.; Wang, Z.-L. Chin. Chem. Lett. 2021, 32, 3632.
doi: 10.1016/j.cclet.2021.04.019 |
| [38] |
Liu, J.; Liu, Q.; Yi, H.; Qin, C.; Bai, R.; Qi, X.; Lan, Y.; Lei, A. Angew. Chem., nt. Ed. 2014, 53, 502.
|
| [39] |
Xie, Q.; Jiang, M.; Chen, X.; Lv, Q.; Yu, B. Chin. J. Org. Chem. 2021, 41, 4575. (in Chinese)
doi: 10.6023/cjoc202109008 |
|
( 谢坤宸, 江铭轩, 陈晓岚, 吕琪妍, 於兵, 有机化学 2021, 41, 4575.)
doi: 10.6023/cjoc202109008 |
|
| [40] |
(a) Monga, A.; Bagchi, S.; Soni, R. K.; Sharma, A. Adv. Synth. Catal. 2020, 362, 2232.
doi: 10.1002/adsc.201901617 |
|
(b) Su, Y.; Zhang, R.; Xue, W.; Liu, X.; Zhao, Y.; Wang, K.-H.; Huang, D.; Huo, C.; Hu, Y. Org. Biomol. Chem. 2020, 18, 1940.
doi: 10.1039/D0OB00086H |
|
|
(c) Zhu, H.-L.; Zeng, F.-L.; Chen, X.-L.; Sun, K.; Li, H.-C.; Yuan, X.-Y.; Qu, L.-B.; Yu, B. Org. Lett. 2021, 23, 2976.
doi: 10.1021/acs.orglett.1c00655 |
|
|
(d) Ji, W. Q.; Tan, H.; Wang, M.; Li, P. H.; Wang, L. Chem. Commun. 2016, 52, 1462.
doi: 10.1039/C5CC08253F |
|
| [41] |
Yang, S.; Tan, H.; Ji, W.; Zhang, X.; Li, P.; Wang, L. Adv. Synth. Catal. 2017, 359, 443.
doi: 10.1002/adsc.201600721 |
| [42] |
Wang, C.; Qiao, J.; Liu, X.; Song, H.; Sun, Z.; Chu, W. J. Org. Chem. 2018, 83, 1422.
doi: 10.1021/acs.joc.7b02979 |
| [43] |
Bai, Q.-F.; Jin, C.; He, J.-Y.; Feng, G. Org. Lett. 2018, 20, 2172.
doi: 10.1021/acs.orglett.8b00449 |
| [44] |
Zhang, X.; Zhu, P.; Zhang, R.; Li, X.; Yao, T. J. Org. Chem. 2020, 85, 9503.
doi: 10.1021/acs.joc.0c00039 |
| [45] |
Shi, W.; Ma, F.; Li, P.; Wang, L.; Miao, T. J. Org. Chem. 2020, 85, 13808.
doi: 10.1021/acs.joc.0c01916 |
| [46] |
Li, J.; Lu, X.-C.; Xu, Y.; Wen, J.-X.; Hou, G.-Q.; Liu, Li. Org. Lett. 2020, 22, 9621.
doi: 10.1021/acs.orglett.0c03663 |
| [47] |
Sun, B.; Shi, R.; Zhang, K.; Tang, X.; Shi, X.; Xu, J.; Yang, J.; Jin, C. Chem. Commun. 2021, 57, 6050.
doi: 10.1039/D1CC02415A |
| [48] |
Yang, B.; Li, S.-J.; Wang, Y.; Lan, Y.; Zhu, S. Nat. Commun. 2021, 12, 5257.
doi: 10.1038/s41467-021-25594-4 pmid: 34489468 |
| [1] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [2] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [3] | 黄芬, 罗维纬, 周俊. 基于C—H键断裂的多氯烷基化反应研究进展[J]. 有机化学, 2023, 43(7): 2368-2390. |
| [4] | 田钰, 张娟, 高文超, 常宏宏. 二甲亚砜作为甲基化试剂在有机合成中的应用[J]. 有机化学, 2023, 43(7): 2391-2406. |
| [5] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [6] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [7] | 李靖鹏, 黄顺桃, 杨棋, 李伟强, 刘腾, 黄超. 利用连续流动技术合成(Z)-N-乙烯基取代N,O-缩醛[J]. 有机化学, 2023, 43(4): 1550-1558. |
| [8] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [9] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [10] | 张妍妍, 张珠珠, 朱圣卿, 储玲玲. 镍催化不对称酰基化反应研究进展[J]. 有机化学, 2023, 43(3): 1023-1035. |
| [11] | 赵金晓, 魏彤辉, 柯森, 李毅. 可见光催化合成二氟烷基取代的多环吲哚化合物[J]. 有机化学, 2023, 43(3): 1102-1114. |
| [12] | 黄嘉为, 李潇漫, 徐亮, 韦玉. α-酮酸与硫酚的电化学脱羧偶联: 一种合成硫代酸酯的新方法[J]. 有机化学, 2023, 43(2): 756-762. |
| [13] | 南宁, 吴双, 秦景灏, 李金恒. 基于硅烷化启动的环化反应研究进展[J]. 有机化学, 2023, 43(10): 3414-3453. |
| [14] | 桑田, 贾帆, 何静, 李春天, 刘岩, 刘平. I2催化β-酮腈与1H-吡唑-5-胺的环化反应[J]. 有机化学, 2023, 43(1): 195-201. |
| [15] | 刘东汉, 鲁席杭, 柴张梦洁, 杨浩琦, 孙瑜琳, 余富朝. 构建2H-吡咯-2-酮骨架的研究进展[J]. 有机化学, 2023, 43(1): 57-73. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
