有机化学 ›› 2026, Vol. 46 ›› Issue (1): 96-105.DOI: 10.6023/cjoc202508010 上一篇 下一篇
研究论文
张元贺a,b, 沈运杰a,b, 谈东兴a,*( ), 韩福社a,b,*(
), 韩福社a,b,*( )
)
Yuanhe Zhanga,b, Yunjie Shena,b, Dongxing Tana,*( ), Fushe Hana,b,*(
), Fushe Hana,b,*( )
)
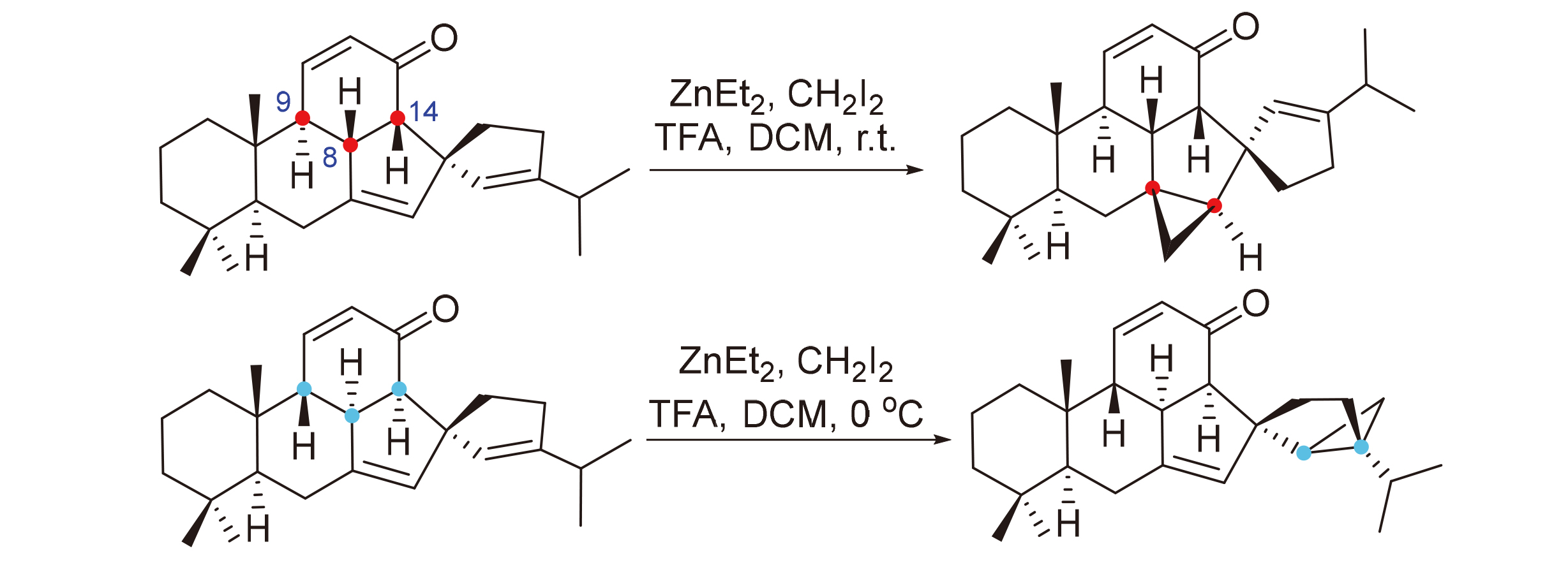
6-6-6-5-5螺五环化合物是本课题组发展的合成含亚甲基苯醌结构的C30萜类天然物及其立体异构体路线中的关键中间体, 几种非对映异构体的立体构型对后期环丙烷化反应的活性和立体选择性有显著影响. 对几种非对映异构体构型影响环丙烷化反应活性和立体选择性的原因进行了详细探究. 首先通过适当的衍生化, 并结合二维核磁分析确定了四个非对映异构体中C9, C8和C14的构型, 据此将异构体的构型与反应活性和选择性一一对应. 随后根据确定的构型, 利用密度泛函(DFT)理论计算对四个异构体的三维结构进行了优化, 从立体效应的角度为解释异构体之间存在反应活性和选择性差异的原因提供了直观证据. 此外, 还利用氘代实验对构建6-6-6-5-5螺并环骨架的分子内Michael/ aldol串联反应的机理进行了探究.