Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (2): 323-343.DOI: 10.6023/cjoc202109017 Previous Articles Next Articles
REVIEWS
程异, 胡荣静*( ), 陈晓琪, 杨浩, 牛晓康, 杨磊*(
), 陈晓琪, 杨浩, 牛晓康, 杨磊*( )
)
收稿日期:2021-09-13
修回日期:2021-10-06
发布日期:2022-02-24
通讯作者:
胡荣静, 杨磊
基金资助:
Yi Cheng, Rongjing Hu( ), Xiaoqi Chen, Hao Yang, Xiaokang Niu, Lei Yang(
), Xiaoqi Chen, Hao Yang, Xiaokang Niu, Lei Yang( )
)
Received:2021-09-13
Revised:2021-10-06
Published:2022-02-24
Contact:
Rongjing Hu, Lei Yang
Supported by:Share
Yi Cheng, Rongjing Hu, Xiaoqi Chen, Hao Yang, Xiaokang Niu, Lei Yang. Recent Progress in Direct Catalytic C(sp3)—H Silylation Reactions[J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 323-343.
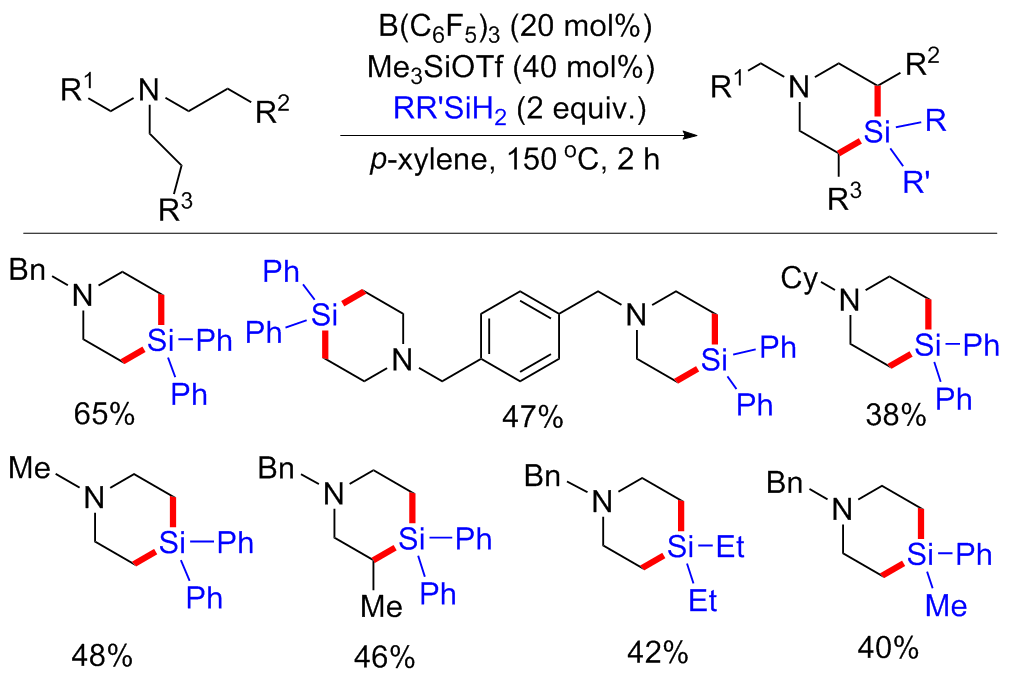
| [1] |
(a) Min, E.-Z.; Wu, W. Green Chemistry and Chemical Engineering, Chemical Industry Press Co. Ltd., Beijing, 2000. (in Chinese)
|
|
( 闵恩泽, 吴巍, 绿色化学与化工, 化学工业出版社, 北京, 2000.)
|
|
|
(b) Ma, S.-M.; Wei, X.-F. Atom Economical Organic Reactions, China Petrochemical Press Co. Lid., Beijing, 2006. (in Chinese)
|
|
|
( 麻生明, 魏晓芳, 原子经济性反应, 中国石化出版社, 北京, 2006.)
|
|
| [2] |
(a) Yu, J.-Q.; Shi, Z.-J. Topics in Current Chemistry, Springer, Berlin, 2010, Vol. 292.
pmid: 30033454 |
|
(b) Chen, Z.; Wang, B.; Zhang, J.; Yu, W.; Liu, Z.; Zhang, Y. Org. Chem. Front. 2015, 2, 1107.
doi: 10.1039/C5QO00004A pmid: 30033454 |
|
|
(c) Hartwig, J. F.; Larsen, M. A. ACS Cent. Sci. 2016, 2, 281.
doi: 10.1021/acscentsci.6b00032 pmid: 30033454 |
|
|
(d) Xu, Y.; Dong, G. Chem. Sci. 2018, 9, 1424.
doi: 10.1039/C7SC04768A pmid: 30033454 |
|
|
(e) Sambiagio, C.; Schönbauer, D.; Blieck, R.; Dao-Huy, T.; Pototschnig, G.; Schaaf, P.; Wiesinger, T.; Zia, M. F.; Wencel-Delord, J.; Besset, T.; Maes, B. U. W.; Schnürch, M. Chem. Soc. Rev. 2018, 47, 6603.
doi: 10.1039/c8cs00201k pmid: 30033454 |
|
| [3] |
(a) Daugulis, O.; Do, H.-Q.; Shabashov, D. Acc. Chem. Res. 2009, 42, 1074.
doi: 10.1021/ar9000058 |
|
(b) Jazzar, R.; Hitce, J.; Renaudat, A.; Sofack-Kreutzer, J.; Baudoin, O. Chem.-Eur. J. 2010, 16, 2654.
doi: 10.1002/chem.200902374 |
|
|
(c) Li, H.; Li, B.-J.; Shi, Z.-J. Catal. Sci. Technol. 2011, 1, 191.
doi: 10.1039/c0cy00076k |
|
| [4] |
(a) He, J.; Wasa, M.; Chan, K. S. L.; Shao, Q.; Yu, J.-Q. Chem. Rev. 2017, 117, 8754.
doi: 10.1021/acs.chemrev.6b00622 |
|
(b) Chen, Z.; Rong, M.-Y.; Nie, J.; Zhu, X.-F.; Shi, B.-F.; Ma, J.-A. Chem. Soc. Rev. 2019, 48, 4921.
doi: 10.1039/C9CS00086K |
|
|
(c) Huang, H.; Li, T.; Wang, J.; Qin, G.; Xiao, T. Chin. J. Org. Chem. 2019, 39, 1511. (in Chinese)
|
|
|
( 黄鸿泰, 李涛, 王家状, 秦贵平, 肖铁波, 有机化学, 2019, 39, 1511.)
doi: 10.6023/cjoc201903078 |
|
|
(d) Das, J.; Guin, S.; Maiti, D. Chem. Sci. 2020, 11, 10887.
doi: 10.1039/D0SC04676K |
|
|
(e) Zhang, Q.; Shi, B.-F. Chem. Sci. 2021, 12, 841.
doi: 10.1039/D0SC05944G |
|
|
(f) Mingo, M. M.; Rodríguez.,
doi: 10.1039/D1QO00389E |
|
| [5] |
(a) Langkopf, E.; Schinzer, D. Chem. Rev. 1995, 95, 1375.
doi: 10.1021/cr00037a011 pmid: 29039662 |
|
(b) Jones, R. G.; Ando, W.; Chojnowski, J. Silicon-Containing Polymers, Springer, Berlin, 2000.
pmid: 29039662 |
|
|
(c) Showell, G. A.; Mills, J. S. Drug Discovery Today 2003, 8, 551.
pmid: 29039662 |
|
|
(d) Franz, A. K. Curr. Opin. Drug Discovery Dev. 2007, 10. 654.
pmid: 29039662 |
|
|
(e) Franz, A. K.; Wilson, S. O. J. Med. Chem. 2013, 56, 388.
doi: 10.1021/jm3010114 pmid: 29039662 |
|
|
(f) Fujii, S.; Hashimoto, Y. Future Med. Chem. 2017, 9, 485.
doi: 10.4155/fmc-2016-0193 pmid: 29039662 |
|
|
(g) Ramesh, R.; Reddy, D. S. J. Med. Chem. 2018, 61, 3779.
doi: 10.1021/acs.jmedchem.7b00718 pmid: 29039662 |
|
|
(h) Sarai, N. S.; Levin, B. J.; Roberts, J. M.; Katsoulis, D. E.; Arnold, F. H. ACS Cent. Sci. 2021, 7, 944.
doi: 10.1021/acscentsci.1c00182 pmid: 29039662 |
|
|
(i) Zuo, Y.; Gou, Z.; Quan, W.; Lin, W. Coord. Chem. Rev. 2021, 438, 213887.
doi: 10.1016/j.ccr.2021.213887 pmid: 29039662 |
|
| [6] |
(a) Brook, M. A. Silicon in Organic, Organometallic, and Polymer Chemistry, Wiley, New York, 2000.
|
|
(b) Bähr, S.; Xue, W.; Oestreich, M. ACS Catal. 2019, 9, 16.
doi: 10.1021/acscatal.8b04230 |
|
| [7] |
(a) Cheng, C.; Hartwig, J. F. Chem. Rev. 2015, 115, 8946.
doi: 10.1021/cr5006414 pmid: 31723309 |
|
(b) Sharma, R.; Kumar, R.; Kumar, I.; Singh, B.; Sharma, U. Synthesis 2015, 2347.
pmid: 31723309 |
|
|
(c) Yang, Y.; Wang, C. Sci. China: Chem. 2015, 58, 1266.
pmid: 31723309 |
|
|
(d) Hartwig, J. F.; Romero, E. A. Tetrahedron 2019, 75, 4059.
doi: 10.1016/j.tet.2019.05.055 pmid: 31723309 |
|
|
(e) Zhou, B.; Lu, A.; Zhang, Y. Synlett 2019, 30, 685.
doi: 10.1055/s-0037-1610339 pmid: 31723309 |
|
|
(f) Richter, S. C.; Oestreich, M. Trends Chem. 2020, 2, 13.
doi: 10.1016/j.trechm.2019.07.003 pmid: 31723309 |
|
|
(g) Li, B.; Dixneuf, P. H. Chem. Soc. Rev. 2021, 50, 5062.
doi: 10.1039/D0CS01392G pmid: 31723309 |
|
| [8] |
(a) Mo, F.; Tabor, J. R.; Dong, G. Chem. Lett. 2014, 43, 264.
doi: 10.1246/cl.131154 |
|
(b) Huang, Z.; Lim, H. N.; Mo, F.; Young, M. C.; Dong, G. Chem. Soc. Rev. 2015, 44, 7764.
doi: 10.1039/C5CS00272A |
|
| [9] |
Simmons, E. M.; Hartwig, J. F. Nature 2012, 483, 70.
doi: 10.1038/nature10785 |
| [10] |
Parija, A.; Sunoj, R. B. Org. Lett. 2013, 15, 4066.
doi: 10.1021/ol401597t |
| [11] |
Mita, T.; Michigami, K.; Sato, Y. Org. Lett. 2012, 14, 3462.
doi: 10.1021/ol301431d |
| [12] |
Mita, T.; Michigami, K.; Sato, Y. Chem.-Asian J. 2013, 8, 2970.
doi: 10.1002/asia.v8.12 |
| [13] |
Frihed, T. G.; Heuckendorff, M.; Pedersen, C. M.; Bols, M. Angew. Chem., Int. Ed. 2012, 51, 12285.
doi: 10.1002/anie.v51.49 |
| [14] |
Li, B.; Driess, M.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 6586.
doi: 10.1021/ja5026479 |
| [15] |
(a) Wang, J. Stereoselective Alkene Synthesis, Springer Verlag, Heidelberg, New York, 2012.
pmid: 33491691 |
|
(b) Zhang, J.; Lu, X.; Shen, C.; Xu, L.; Ding, L.; Zhong, G. Chem. Soc. Rev. 2021, 50, 3263.
doi: 10.1039/d0cs00447b pmid: 33491691 |
|
| [16] |
Ghavtadze, N.; Melkonyan, F. S.; Gulevich, A. V.; Huang, C.; Gevorgyan, V. Nat. Chem. 2014, 6, 122.
doi: 10.1038/nchem.1841 pmid: 24451587 |
| [17] |
Zaitsev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127, 13154
doi: 10.1021/ja054549f |
| [18] |
Li, Q.; Driess, M.; Hartwig, J. F. Angew. Chem., Int. Ed. 2014, 53, 8471.
doi: 10.1002/anie.v53.32 |
| [19] |
Marciniec, B. Coord. Chem. Rev. 2005, 249, 2374.
doi: 10.1016/j.ccr.2005.02.025 |
| [20] |
Jia, X.; Huang, Z. Nat. Chem. 2016, 8, 157.
doi: 10.1038/nchem.2417 |
| [21] |
(a) Fukumoto, Y.; Hirano, M.; Chatani, N. ACS Catal. 2017, 7, 3152.
doi: 10.1021/acscatal.7b00539 pmid: 29131615 |
|
(b) Fukumoto, Y.; Hirano, M.; Matsubara, N.; Chatani, N. J. Org. Chem. 2017, 82, 13649.
doi: 10.1021/acs.joc.7b02375 pmid: 29131615 |
|
| [22] |
Hirano, M.; Fukumoto, Y.; Matsubara, N.; Chatani, N. Chem. Lett. 2018, 47, 385.
doi: 10.1246/cl.171137 |
| [23] |
Su, B.; Hartwig, J. F. J. Am. Chem. Soc. 2017, 139, 12137.
doi: 10.1021/jacs.7b06679 |
| [24] |
Zhang, M.; Liang, J.; Huang, G. J. Org. Chem. 2019, 84, 2372.
doi: 10.1021/acs.joc.9b00117 pmid: 30668096 |
| [25] |
Bunescu, A.; Butcher, T. W.; Hartwig, J. F. J. Am. Chem. Soc. 2018, 140, 1502.
doi: 10.1021/jacs.7b12150 pmid: 29283571 |
| [26] |
He, C.; Whitehurst, W. G.; Gaunt, M. J. Chem 2019, 5, 1031.
doi: 10.1016/j.chempr.2018.12.017 |
| [27] |
Su, B.; Lee, T.; Hartwig, J. F. J. Am. Chem. Soc. 2018, 140, 18032.
doi: 10.1021/jacs.8b10428 |
| [28] |
Yan, Z.-B.; Peng, M.; Chen, Q.-L.; Lu, K.; Tu, Y.-Q.; Dai, K.-L.; Zhang, F.-M.; Zhang, X.-M. Chem. Sci. 2021, 12, 9748.
doi: 10.1039/D1SC02344F |
| [29] |
Kakiuchi, F.; Tsuchiya, K.; Matsumoto, M.; Mizushima, E.; Chatani, N. J. Am. Chem. Soc. 2004, 126, 12792.
doi: 10.1021/ja047040d |
| [30] |
Li, W.; Huang, X.; You, J. Org. Lett. 2016, 18, 666.
doi: 10.1021/acs.orglett.5b03593 |
| [31] |
Kon, K.; Suzuki, H.; Takada, K.; Kohari, Y.; Namikoshi, T.; Watanabe, S.; Murata, M. ChemCatChem 2016, 8, 2202.
doi: 10.1002/cctc.v8.13 |
| [32] |
Liu, S.; Lin, Q.; Liao, C.; Chen, J.; Zhang, K.; Liu, Q.; Li, B. Org. Biomol. Chem. 2019, 17, 4115.
doi: 10.1039/C9OB00609E |
| [33] |
van Koten, G.; Milstein, D. Organometallic Pincer Chemistry, Springer Berlin, Heidelberg, 2013.
|
| [34] |
Fang, H.; Hou, W.; Liu, G.; Huang Z. J. Am. Chem. Soc. 2017, 139, 11601.
doi: 10.1021/jacs.7b06798 |
| [35] |
Wen, J.; Dong, B.; Zhu, J.; Zhao, Y.; Shi, Z. Angew. Chem., Int. Ed. 2020, 59, 10909.
doi: 10.1002/anie.v59.27 |
| [36] |
Sakakura, T.; Tokunaga, Y.; Sodeyama, T.; Tanaka, M. Chem. Lett. 1987, 2375.
|
| [37] |
Ureshino, T.; Yoshida, T.; Kuninobu, Y.; Takai, K. J. Am. Chem. Soc. 2010, 132, 14324.
doi: 10.1021/ja107698p |
| [38] |
Kuninobu, Y.; Nakahara, T.; Takeshima, H.; Takai, K. Org. Lett. 2013, 15, 426.
doi: 10.1021/ol303353m |
| [39] |
Murai, M.; Takeshima, H.; Morita, H.; Kuninobu, Y.; Takai, K. J. Org. Chem. 2015, 80, 5407.
doi: 10.1021/acs.joc.5b00920 |
| [40] |
Hua, Y.; Jung, S.; Roh, J.; Jeon, J. J. Org. Chem. 2015, 80, 4661.
doi: 10.1021/acs.joc.5b00564 |
| [41] |
Lee, T.; Hartwig, J. F. Angew. Chem., Int. Ed. 2016, 55, 8723.
doi: 10.1002/anie.v55.30 |
| [42] |
Karmel, C.; Li, B.; Hartwig, J. F. J. Am. Chem. Soc. 2018, 140, 1460.
doi: 10.1021/jacs.7b11964 pmid: 29293327 |
| [43] |
Xu, L.-W. In Organosilicon Compounds: From Theory to Synthesis to Applications, Ed.: Lee, V. Y., Elsevier, London, 2017, Vol. 1, pp 145-194.
|
| [44] |
(a) Yang, B.; Yang, W.; Guo, Y.; You, L.; He, C. Angew. Chem., Int. Ed. 2020, 59, 22217.
doi: 10.1002/anie.v59.49 |
|
(b) Guo, Y.; Liu, M.-M.; Zhu, X.; Zhu, L.; He, C. Angew. Chem., Int. Ed. 2021, 60, 13887.
doi: 10.1002/anie.v60.25 |
|
| [45] |
Xiao, P.; Gao, L.; Song, Z. Chem.-Eur. J. 2019, 25, 2407.
doi: 10.1002/chem.v25.10 |
| [46] |
(a) Larsson, J. M.; Zhao, T. S. N.; Szabó, K. J. Org. Lett. 2011, 13, 1888.
doi: 10.1021/ol200445b |
|
(b) Nakai, S.; Matsui, M.; Shimizu, Y.; Adachi, Y.; Obora, Y. J. Org. Chem. 2015, 80, 7317.
doi: 10.1021/acs.joc.5b01216 |
|
| [47] |
Kanyiva, K. S.; Kuninobu, Y.; Kanai, M. Org. Lett. 2014, 16, 1968.
doi: 10.1021/ol500519y |
| [48] |
Chen, C.; Guan, M.; Zhang, J.; Wen, Z.; Zhao, Y. Org. Lett. 2015, 17, 3646.
doi: 10.1021/acs.orglett.5b01393 |
| [49] |
Liu, Y.-J.; Liu, Y.-H.; Zhang, Z.-Z.; Yan, S.-Y.; Chen, K.; Shi, B.-F. Angew. Chem., Int. Ed. 2016, 55, 13859.
doi: 10.1002/anie.201607766 |
| [50] |
Pan, J.-L.; Li, Q.-Z.; Zhang, T.-Y.; Hou, S.-H.; Kang, J.-C.; Zhang, S.-Y. Chem. Commun. 2016, 52, 13151.
doi: 10.1039/C6CC07885K |
| [51] |
Deb, A.; Singh, S.; Seth, K.; Pimparkar, S.; Bhaskararao, B.; Guin, S.; Sunoj, R. B.; Maiti, D. ACS Catal. 2017, 7, 8171.
doi: 10.1021/acscatal.7b03056 |
| [52] |
Lu, A.; Ji, X.; Zhou, B.; Wu, Z.; Zhang, Y. Angew. Chem.,Int. Ed. 2018, 57, 3233.
doi: 10.1002/anie.201800330 |
| [53] |
Rémond, E.; Martin, C.; Martinez, J.; Cavelier, F. Chem. Rev. 2016. 116. 11654.
doi: 10.1021/acs.chemrev.6b00122 |
| [54] |
Zhan, B.-B.; Fan, J.; Jin, L.; Shi, B.-F. ACS Catal. 2019, 9, 3298.
doi: 10.1021/acscatal.9b00544 |
| [55] |
Li, J.; Jiang, C. Org. Lett. 2021, 23, 5359.
doi: 10.1021/acs.orglett.1c01678 |
| [56] |
Tsukada, N.; Hartwig, J. F. J. Am. Chem. Soc. 2005, 127, 5022.
doi: 10.1021/ja050612p |
| [57] |
Liu, J.; Zhang, W.; Xi, Z. Chin. J. Org. Chem. 2009, 29, 491. (in Chinese)
|
|
( 刘俊辉, 张文雄, 席振峰, 有机化学, 2009, 29, 491.)
|
|
| [58] |
Ishikawa, M.; Okazaki, S.; Naka, A.; Sakamoto, H. Organometallics 1992, 11, 4135.
doi: 10.1021/om00060a033 |
| [59] |
(a) Baba, T.; Kato, A.; Yuasa, H.; Toriyama, F.; Handa, H.; Ona, Y. Catal. Today 1998, 44, 271.
doi: 10.1016/S0920-5861(98)00199-0 |
|
(b) Toutov, A. A.; Liu, W.-B.; Betz, K. M.; Fedorov, A.; Stoltz, B. M.; Grubbs, R. H. Nature 2015, 518, 80.
doi: 10.1038/nature14126 |
|
| [60] |
Harder, S. Chem. Rev. 2010, 110, 3852.
doi: 10.1021/cr9003659 |
| [61] |
Zhao, L. X.; Shi, X. H.; Cheng, J. H. ACS Catal. 2021, 11, 2041.
doi: 10.1021/acscatal.0c05440 |
| [62] |
(a) Zhang, J.; Park, S.; Chang, S. J. Am. Chem. Soc. 2018, 140, 13209.
doi: 10.1021/jacs.8b08733 |
|
(b) Zhou, M.; Park, S.; Dang, L. Org. Chem. Front. 2020, 7, 944.
doi: 10.1039/C9QO01437C |
|
|
(c) Zhang, J.; Chang, S. J. Am. Chem. Soc. 2020, 142, 12585.
doi: 10.1021/jacs.0c05241 |
|
| [63] |
Fang, H.; Xie, K.; Kemper, S.; Oestreich, M. Angew. Chem., Int. Ed. 2021, 60, 8542.
doi: 10.1002/anie.v60.15 |
| [64] |
Ihara, H.; Ueda, A.; Suginome, M. Chem. Lett. 2011, 40, 916.
doi: 10.1246/cl.2011.916 |
| [65] |
Liu, P.; Tang, J.; Zeng, X. Org. Lett. 2016, 18, 5536.
doi: 10.1021/acs.orglett.6b02784 |
| [66] |
Feng, J.-J.; Oestreich, M. Org. Lett. 2018, 20, 4273.
doi: 10.1021/acs.orglett.8b01698 |
| [67] |
Zhang, X.; Geng, P.; Liu, G.; Huang, Z. Organometallics 2021, 40, 2365.
doi: 10.1021/acs.organomet.1c00264 |
| [68] |
Luo, Y.; Teng, H.-L.; Xue, C.; Nishiura, M.; Hou, Z. ACS Catal. 2018, 8, 8027.
doi: 10.1021/acscatal.8b02405 |
| [69] |
Gentle, T. M.; Muetterties, E. L. J. Am. Chem. Soc. 1983, 105, 304.
doi: 10.1021/ja00340a032 |
| [70] |
(a) Procopio, L. J.; Berry, D. H. J. Am. Chem. Soc. 1991, 113, 4039.
doi: 10.1021/ja00010a081 |
| [71] |
( b, Djurovich, P. I.; Dolich, A. R.; Berry, D. H. J. Chem. Soc. Chem. Commun. 1994, 1897. (c) Ezbiansky, K.; Djurovich, P. I.; LaForest, M.; Sinning, D. J. Zayes, R.; Berry, D. H. Organometallics 1998, 17, 1455.
|
| [72] |
Murai, M.; Takeuchi, Y.; Takai, K. Chem. Lett. 2017, 46, 1044.
doi: 10.1246/cl.170369 |
| [73] |
Gunsalus, N. J.; Koppaka, A.; Park, S. H.; Bischof, S. M.; Hashiguchi, B. G.; Periana, R. A. Chem. Rev. 2017, 117, 8521.
doi: 10.1021/acs.chemrev.6b00739 pmid: 28459540 |
| [74] |
(a) Sadow, A. D.; Tilley, T. D. Angew. Chem., Int. Ed. 2003, 42, 803.
doi: 10.1002/anie.200390213 |
|
(b) Sadow, A. D.; Tilley, T. D. J. Am. Chem. Soc. 2005, 127, 643.
doi: 10.1021/ja040141r |
| [1] | Jianghu Dong, Liangming Xuan, Chi Wang, Chenxi Zhao, Haifeng Wang, Qiongjiao Yan, Wei Wang, Fen'er Chen. Recent Advances in Visible-Light-Induced C(3)—H Functionalization of Quinoxalinones under Transition-Metal-Free or Photocatalyst-Free [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 111-136. |
| [2] | Xinqiang Chen, Jing Zhang. Progress in the Study of Dehydroxymethylation of Primary Alcohol [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2711-2719. |
| [3] | Yijun Shi, Xinyue Sun, Han Cao, Fusheng Bie, Jie Ma, Zhe Liu, Xingshun Cong. Thioesterification of Esters with Primary Aliphatic Thiols at Room Temperature [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2499-2505. |
| [4] | Mingyang Pang, Honghong Chang, Zhang Feng, Juan Zhang. Recent Advances in Transition-Metal-Catalyzed Tandem Dearomatization of Indoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1271-1291. |
| [5] | Ling Meng, Jun Wang. Research Progress on Synthesis of Thioflavonoids [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 873-891. |
| [6] | Rui Wang, Lang Gao, Cen Zhou, Xiao Zhang. Haloperfluoroalkylation of Unactivated Terminal Alkenes over Phenylphenothiazine-Based Porous Organic Polymers [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1136-1145. |
| [7] | Sining Qin. Research Progress in C—S Coupling Reactions of Aryl Halides [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3761-3783. |
| [8] | Yan Zeng, Fei Ye. Research Progress on New Catalytic Reaction Systems for Asymmetric Synthesis of Silicon-Stereogenic Center Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3388-3413. |
| [9] | Qiyang Li, Haiyan Zhang, Wenbo Liu. Research Progress in Transition-Metal-Free C—Si Bond Formation [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3470-3490. |
| [10] | Cheng Yuan, Changduo Pan. Recent Advances in the N-Aryl C—H Functionalization Using 7-Azaindole as Intrinsic Directing Group [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 156-170. |
| [11] | Jinyu Zhang, Tianfen Liu, Le Wang, Xiaoming Wang. Recent Process in the in situ Generated Metal Nanocluster Catalysis [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2331-2341. |
| [12] | Cheng Gong, Jian Tang, Fei Xu, Pengjie Li, Zetian Wang, Yumin Zhang, Guoxian Yu, Liang Wang. Recent Advances in Transition-Metal-Catalyzed C—H Activation of Pyridone/Isoquinolones [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 1925-1949. |
| [13] | Yazhou Wang, Yuhang Zhu, Lixia Xu, Rui He, Jian Zhang. Recent Advances in Geminal-Group-Directed Alkenyl C—H Functionalization [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2000-2014. |
| [14] | Siqi Cong, Mengya Liu, Siyuan Peng, Qiucui Zheng, Mengjiao Li, Yan Guo, Feixian Luo. Cross-Coupling of C—Si Bond by Using of Silyl Electrophiles [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 384-390. |
| [15] | Yaohui Xu, Zhen Wu, Xinxin Wu, Chen Zhu. Transition-Metal Free Radical-Mediated C—H Bond Alkynylation and Allylation of Ethers, Aldehydes and Amides [J]. Chinese Journal of Organic Chemistry, 2022, 42(12): 4340-4349. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||