Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (3): 679-697.DOI: 10.6023/cjoc202110009 Previous Articles Next Articles
REVIEWS
收稿日期:2021-10-09
修回日期:2021-11-13
发布日期:2021-11-25
通讯作者:
杨晓瑜
基金资助:
Yunrong Chen, Wei Liu, Xiaoyu Yang( )
)
Received:2021-10-09
Revised:2021-11-13
Published:2021-11-25
Contact:
Xiaoyu Yang
Supported by:Share
Yunrong Chen, Wei Liu, Xiaoyu Yang. Recent Advances in Kinetic Resolution of Tertiary Alcohols[J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 679-697.
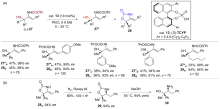
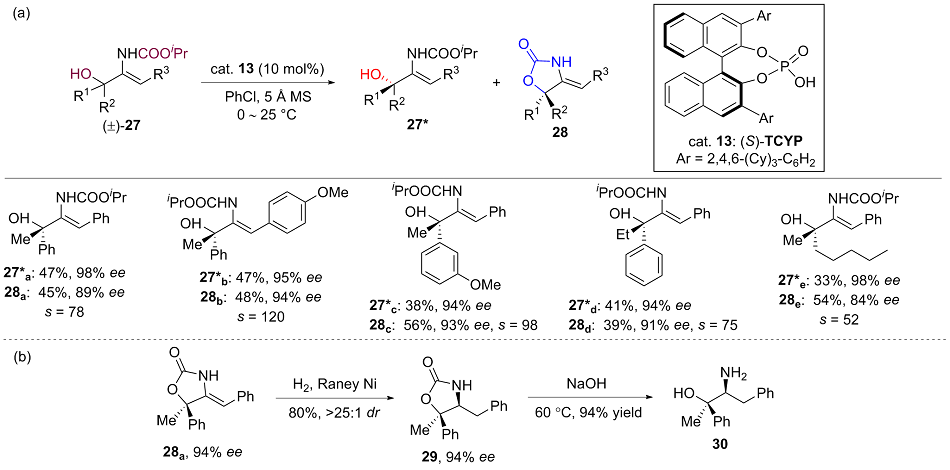
| [1] |
(a) Shibasaki, M.; Kanai, M. Chem. Rev. 2008, 108 (8), 2853.
doi: 10.1021/cr078340r |
|
(b) Liu, Y.-L.; Lin, X.-T. Adv. Syn. Catal. 2019, 361 (5), 876.
doi: 10.1002/adsc.v361.5 |
|
|
(c) Collados, J. F.; Solà, R.; Harutyunyan, S. R.; Maciá, B. ACS Catal. 2016, 6 (3), 1952.
doi: 10.1021/acscatal.5b02832 |
|
|
(d) Rong, J.; Pellegrini, T.; Harutyunyan, S. R. Chem.-Eur. J. 2016, 22 (11), 3558.
doi: 10.1002/chem.201503412 |
|
|
(e) Zhu, D.; Xu, M.-H. Chin. J. Org. Chem. 2020, 40, 255. (in Chinese)
doi: 10.6023/cjoc201910009 |
|
|
(祝东星, 徐明华, 有机化学, 2020, 40, 255.)
|
|
|
(f) Luo, R.; Liao, J.; Zhang, J. Chin. J. Org. Chem. 2013, 33, 2298. (in Chinese)
doi: 10.6023/cjoc201305044 |
|
|
(罗人仕, 廖建华, 张剑, 有机化学, 2013, 33, 2298.)
|
|
|
(g) Fu, Y.; Hou, B.; Zhao, X.; Du, Z.; Hu, Y. Chin. J. Org. Chem. 2015, 35, 2507. (in Chinese)
doi: 10.6023/cjoc201505045 |
|
|
(傅颖, 侯博, 赵兴玲, 杜正银, 胡雨来, 有机化学, 2015, 35, 2507.)
doi: 10.6023/cjoc201505045 |
|
| [2] |
(a) Sim, S.-B. D.; Wang, M.; Zhao, Y. ACS Catal. 2015, 5(6), 3609.
doi: 10.1021/acscatal.5b00583 |
|
(b) Bergonzini, G.; Melchiorre, P. Angew. Chem. Int. Ed. 2012, 51(4), 971.
doi: 10.1002/anie.v51.4 |
|
|
(c) Jung, B.; Hong, M. S.; Kang, S. H. Angew. Chem. Int. Ed. 2007, 46(15), 2616.
doi: 10.1002/(ISSN)1521-3773 |
|
| [3] |
(a) Robinson, D. E. J. E.; Bull, S. D. Tetrahedron: Asymmetry 2003, 14(11), 1407.
doi: 10.1016/S0957-4166(03)00209-X |
|
(b) Vedejs, E.; Jure, M. Angew. Chem. Int. Ed. 2005, 44(26), 3974.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(c) Müller, C. E.; Schreiner, P. R. Angew. Chem. Int. Ed. 2011, 50(27), 6012.
doi: 10.1002/anie.v50.27 |
|
|
(d) Pellissier, H. Adv. Syn. Catal. 2011, 353(10), 1613.
doi: 10.1002/adsc.201100111 |
|
|
(e) Krasnov, V. P.; Gruzdev, D. A.; Levit, G. L. Eur. J. Org. Chem. 2012, 2012(8), 1471.
doi: 10.1002/ejoc.v2012.8 |
|
|
(f) Petersen, K. S. Asian J. Org. Chem. 2016, 5(3), 308.
doi: 10.1002/ajoc.v5.3 |
|
|
(g) Chen, S.; Shi, Y.-H.; Wang, M. Chem. Asian J. 2018, 13(17), 2184.
doi: 10.1002/asia.201800537 |
|
|
(h) Wang, Z.; Pan, D.; Li, T.; Jin, Z. Chem. Asian J. 2018, 13(17), 2149.
doi: 10.1002/asia.201800493 |
|
|
(i) Yang, H.; Zheng, W.-H. Tetrahedron Lett. 2018, 59(7), 583.
doi: 10.1016/j.tetlet.2017.12.080 |
|
|
(j) Liu, W.; Yang, X. Asian J. Org. Chem. 2021, 10(4), 692.
doi: 10.1002/ajoc.v10.4 |
|
| [4] |
Kagna, H. B.; Fiaud, J. C., Topics in Stereochemistry. In Kinetic Resolution, Eliel, E. L.; Wilen, S. H., Eds. Wiley; New York: 1988; Vol. 18, p. 249.
|
| [5] |
(a) Özdemirhan, D. Synth. Commun. 2017, 47(7), 629.
doi: 10.1080/00397911.2016.1274032 |
|
(b) Özdemirhan, D.; Sezer, S.; Sönmez, Y. Tetrahedron: Asymmetry 2008, 19(23), 2717.
doi: 10.1016/j.tetasy.2008.12.002 |
|
|
(c) Deng, D.; Zhang, Y.; Sun, A.; Sai, K.; Hu, Y. Chin. J. Org. Chem. 2018, 38, 1185. (in Chinese)
doi: 10.6023/cjoc201710019 |
|
|
(邓盾, 张云, 孙爱君, 赛克, 胡云峰, 有机化学, 2018, 38, 1185.)
doi: 10.6023/cjoc201710019 |
|
| [6] |
Jarvo, E. R.; Evans, C. A.; Copeland, G. T.; Miller, S. J. J. Org. Chem. 2001, 66(16), 5522.
pmid: 11485477 |
| [7] |
Angione, M. C.; Miller, S. J. Tetrahedron 2006, 62(22), 5254.
doi: 10.1016/j.tet.2006.01.104 |
| [8] |
(a) Lu, S.; Poh, S. B.; Siau, W.-Y.; Zhao, Y. Angew. Chem., nt. Ed. 2013, 52(6), 1731.
|
|
(b) Lu, S.; Poh, S. B.; Siau, W.-Y.; Zhao, Y. Synlett 2013, 24(10), 1165.
doi: 10.1055/s-00000083 |
|
| [9] |
Greenhalgh, M. D.; Smith, S. M.; Walden, D. M.; Taylor, J. E.; Brice, Z.; Robinson, E. R. T.; Fallan, C.; Cordes, D. B.; Slawin, A. M. Z.; Richardson, H. C.; Grove, M. A.; Cheong, P. H.-Y.; Smith, A. D. Angew. Chem. Int. Ed. 2018, 57(12), 3200.
doi: 10.1002/anie.v57.12 |
| [10] |
Guha, N. R.; Neyyappadath, R. M.; Greenhalgh, M. D.; Chisholm, R.; Smith, S. M.; McEvoy, M. L.; Young, C. M.; Rodríguez- Escrich, C.; Pericàs, M. A.; Hähner, G.; Smith, A. D. Green Chem. 2018, 20(19), 4537.
doi: 10.1039/C8GC02020E |
| [11] |
Young, C. M.; Elmi, A.; Pascoe, D. J.; Morris, R. K.; McLaughlin, C.; Woods, A. M.; Frost, A. B.; de la Houpliere, A.; Ling, K. B.; Smith, T. K.; Slawin, A. M. Z.; Willoughby, P. H.; Cockroft, S. L.; Smith, A. D. Angew. Chem. Int. Ed. 2020, 59(9), 3705.
doi: 10.1002/anie.v59.9 |
| [12] |
Qu, S.; Smith, S. M.; Laina-Martín, V.; Neyyappadath, R. M.; Greenhalgh, M. D.; Smith, A. D. Angew. Chem. Int. Ed. 2020, 59(38), 16572.
doi: 10.1002/anie.v59.38 |
| [13] |
Mandai, H.; Shiomoto, R.; Fujii, K.; Mitsudo, K.; Suga, S. Org. Lett. 2021, 23(4), 1169.
doi: 10.1021/acs.orglett.0c03956 |
| [14] |
Karatas, B.; Rendler, S.; Fröhlich, R.; Oestreich, M. Org. Biomol. Chem. 2008, 6(8), 1435.
doi: 10.1039/b802186d pmid: 18385850 |
| [15] |
Seliger, J.; Dong, X.; Oestreich, M. Angew. Chem. Int. Ed. 2019, 58(7), 1970.
doi: 10.1002/anie.v58.7 |
| [16] |
Čorić, I.; Müller, S.; List, B. J. Am. Chem. Soc. 2010, 132(49), 17370.
doi: 10.1021/ja108642s |
| [17] |
Yamanaka, T.; Kondoh, A.; Terada, M. J. Am. Chem. Soc. 2015, 137(3), 1048.
doi: 10.1021/ja512238n pmid: 25581575 |
| [18] |
Kim, J. H.; Čorić, I.; Palumbo, C.; List, B. J. Am. Chem. Soc. 2015, 137(5), 1778.
doi: 10.1021/ja512481d |
| [19] |
Yoneda, N.; Matsumoto, A.; Asano, K.; Matsubara, S. Chem. Lett. 2016, 45(11), 1300.
doi: 10.1246/cl.160727 |
| [20] |
Rajkumar, S.; He, S.; Yang, X. Angew. Chem. Int. Ed. 2019, 58(30), 10315.
doi: 10.1002/anie.v58.30 |
| [21] |
Rajkumar, S.; Tang, M.; Yang, X. Angew. Chem. Int. Ed. 2020, 59(6), 2333.
doi: 10.1002/anie.v59.6 |
| [22] |
Pan, Y.; Jiang, Q.; Rajkumar, S.; Zhu, C.; Xie, J.; Yu, S.; Chen, Y.; He, Y. P.; Yang, X. Adv. Syn. Catal. 2020, 363(1), 200.
doi: 10.1002/adsc.v363.1 |
| [23] |
Zheng, Z.; Cao, Y.; Chong, Q.; Han, Z.; Ding, J.; Luo, C.; Wang, Z.; Zhu, D.; Zhou, Q. L.; Ding, K. J. Am. Chem. Soc. 2018, 140(32), 10374.
doi: 10.1021/jacs.8b07125 |
| [24] |
Zhang, C. H.; Gao, Q.; Li, M.; Wang, J. F.; Yu, C. M.; Mao, B. Org. Lett. 2021, 23(10), 3949.
doi: 10.1021/acs.orglett.1c01110 |
| [25] |
Hua, Y.; Liu, Z.-S.; Xie, P.-P.; Ding, B.; Cheng, H.-G.; Hong, X.; Zhou, Q. Angew. Chem. Int. Ed. 2021, 60(23), 12824.
doi: 10.1002/anie.v60.23 |
| [26] |
Schipper, D. J.; Rousseaux, S.; Fagnou, K. Angew. Chem. Int. Ed. 2009, 48(44), 8343.
doi: 10.1002/anie.200902373 |
| [27] |
Zhao, Y.; Mitra, A. W.; Hoveyda, A. H.; Snapper, M. L. Angew. Chem. Int. Ed. 2007, 46(44), 8471.
doi: 10.1002/(ISSN)1521-3773 |
| [28] |
Olivares-Romero, J. L.; Li, Z.; Yamamoto, H. J. Am. Chem. Soc. 2013, 135(9), 3411.
doi: 10.1021/ja401182a pmid: 23406082 |
| [29] |
Pawliczek, M.; Hashimoto, T.; Maruoka, K. Chem. Sci. 2018, 9(5), 1231.
doi: 10.1039/c7sc04854h pmid: 29675168 |
| [30] |
Huang, B.; He, Y.; Levin, M. D.; Coelho, J. A. S.; Bergman, R. G.; Toste, F. D. Adv. Synth. Catal. 2020, 362(2), 295.
doi: 10.1002/adsc.v362.2 |
| [31] |
Niu, S.; Zhang, H.; Xu, W.; Bagdi, P. R.; Zhang, G.; Liu, J.; Yang, S.; Fang, X. Nat. Commun. 2021, 12(1), 3735.
doi: 10.1038/s41467-021-23990-4 |
| [32] |
Desrues, T.; Liu, X.; Pons, J.-M.; Monnier, V.; Amalian, J.-A.; Charles, L.; Quintard, A.; Bressy, C. Org. Lett. 2021, 23(11), 4332.
doi: 10.1021/acs.orglett.1c01261 |
| [33] |
Tang, M.; Gu, H.; He, S.; Rajkumar, S.; Yang, X. Angew. Chem. Int. Ed. 2021, 60(39), 21334.
doi: 10.1002/anie.v60.39 |
| [34] |
Xie, S.; Gao, X.; Zhou, F.; Wu, H.; Zhou, J. Chin. Chem. Lett. 2020, 31(2), 324.
doi: 10.1016/j.cclet.2019.05.060 |
| [35] |
Liao, K.; Gong, Y.; Zhu, R.-Y.; Wang, C.; Zhou, F.; Zhou, J. Angew. Chem. Int. Ed. 2021, 60(15), 8488.
doi: 10.1002/anie.v60.15 |
| [36] |
Tosaki, S.-y.; Hara, K.; Gnanadesikan, V.; Morimoto, H.; Harada, S.; Sugita, M.; Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2006, 128(36), 11776.
doi: 10.1021/ja064858l |
| [37] |
Hara, K.; Tosaki, S.-y.; Gnanadesikan, V.; Morimoto, H.; Harada, S.; Sugita, M.; Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. Tetrahedron 2009, 65(26), 5030.
doi: 10.1016/j.tet.2009.02.031 |
| [38] |
Shintani, R.; Takatsu, K.; Hayashi, T. Org. Lett. 2008, 10(6), 1191.
doi: 10.1021/ol800120p pmid: 18303902 |
| [39] |
Zhang, W.; Ma, S. Chem. Commun. 2018, 54(47), 6064.
doi: 10.1039/C8CC01949E |
| [40] |
Zheng, W.-F.; Zhang, W.; Huang, C.; Wu, P.; Qian, H.; Wang, L.; Guo, Y.-L.; Ma, S. Nat. Catal. 2019, 2(11), 997.
doi: 10.1038/s41929-019-0346-z |
| [41] |
Wang, J.; Zhang, W.; Wu, P.; Huang, C.; Zheng, Y.; Zheng, W.-F.; Qian, H.; Ma, S. Org. Chem. Front. 2020, 7(23), 3907.
doi: 10.1039/D0QO01106A |
| [42] |
Mao, R.; Zhao, Y.; Zhu, X.; Wang, F.; Deng, W.-Q.; Li, X. Org. Lett. 2021, 23(18), 7038.
doi: 10.1021/acs.orglett.1c02398 |
| [43] |
Kühn, F.; Katsuragi, S.; Oki, Y.; Scholz, C.; Akai, S.; Gröger, H. Chem. Commun. 2020, 56(19), 2885.
doi: 10.1039/C9CC09103C |
| [1] | Shuang Yang, Xinqiang Fang. Kinetic Resolutions Enabled by N-Heterocyclic Carbene Catalysis: An Update [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 448-480. |
| [2] | Wanting Chen, Xiongwei Zhong, Jiale Xing, Changshu Wu, Yang Gao. Progress in Asymmetric Catalytic Synthesis of C—N Axis Chiral Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 349-377. |
| [3] | Quanbin Jiang. Progress in Synthesis of Axially Chiral Compounds through aza-Vinylidene o-Quinone Methide Intermediates [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 159-172. |
| [4] | Chun-Xia Cheng, Lu-Ping Wu, Feng Sha, Xin-Yan Wu. Enantioselective Vinylogous Allylic Alkylation of Coumarins with Morita-Baylis-Hillman Carbonates Catalyzed by Chiral Phosphine-Amide [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3188-3195. |
| [5] | Huijuan Hu, Qiaoli Yan, Xiaogang Lu, Qifan Yang, Chengxin Pei, Hongmei Wang, Runli Gao. Kinetic Resolution of Racemic P-Chiral α-Hydroxymethylphos-phonates Catalyzed by Lipase from Porcine Pancreas [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2815-2825. |
| [6] | Yangyang Chu, Zhaobin Han, Kuiling Ding. Progresses in the Application of Kinetic Resolution in Transition Metal Catalyzed Asymmetric (Transfer) Hydrogenation [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1934-1951. |
| [7] | Cheng Luo, Yanli Yin, Zhiyong Jiang. Recent Advances in Asymmetric Synthesis of P-Chiral Phosphine Oxides [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1963-1976. |
| [8] | Haiqing Wang, Shuang Yang, Yuchen Zhang, Feng Shi. Advances in Catalytic Asymmetric Reactions Involving o-Hydroxybenzyl Alcohols [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 974-999. |
| [9] | Weidi Cao, Xiaohua Liu. Recent Advances on Catalytic Enantioselective Protonation for Construction of α-Tertiary Carbonyl Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 961-973. |
| [10] | Siqiang Fang, Zanjiao Liu, Tianli Wang. Recent Advances of the Atherton-Todd Reaction [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 1069-1083. |
| [11] | Yuliang Chen, Fengkai He, Siyun Wang, Dingcheng Jia, Yaqun Liu, Yiyong Huang. Kinetic Resolution of Aldehydes Bearing an All-Carbon Quaternary Stereocenter at the α-Position by the Antilla Allylboration [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4294-4302. |
| [12] | Jiayi Zhao, Yicong Ge, Chuan He. Construction of Silicon-Stereogenic Center via Catalytic Asymmetric Si—H/X—H Dehydrogenative Coupling [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3352-3366. |
| [13] | Yan Zeng, Fei Ye. Research Progress on New Catalytic Reaction Systems for Asymmetric Synthesis of Silicon-Stereogenic Center Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3388-3413. |
| [14] | Xin Kuang, Changhua Ding, Yichen Wu, Peng Wang. Catalytic Enantioselective Preparation of Chiral Allylsilanes [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3367-3387. |
| [15] | Zengjin Dai, Xumu Zhang, Qin Yin. Advances on Asymmetric Reductive Amination with Ammonium Salts as Amine Sources [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2261-2274. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||