Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (11): 3776-3783.DOI: 10.6023/cjoc202204038 Previous Articles Next Articles
ARTICLES
谭佳敏a, 余雅君a, 关猛a, 赵云辉a,*( ), 唐子龙b, 周智华a, 郭涛c,*(
), 唐子龙b, 周智华a, 郭涛c,*( )
)
收稿日期:2022-04-15
修回日期:2022-06-18
发布日期:2022-07-13
通讯作者:
赵云辉, 郭涛
基金资助:
Jiamin Tana, Yajun Yua, Meng Guana, Yunhui Zhaoa( ), Zilong Tangb, Zhihua Zhoua, Tao Guoc(
), Zilong Tangb, Zhihua Zhoua, Tao Guoc( )
)
Received:2022-04-15
Revised:2022-06-18
Published:2022-07-13
Contact:
Yunhui Zhao, Tao Guo
Supported by:Share
Jiamin Tan, Yajun Yu, Meng Guan, Yunhui Zhao, Zilong Tang, Zhihua Zhou, Tao Guo. Design and Synthesis of Novel Aggregation-Induced Luminescence Molecules Based on Isoquinoline[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3776-3783.

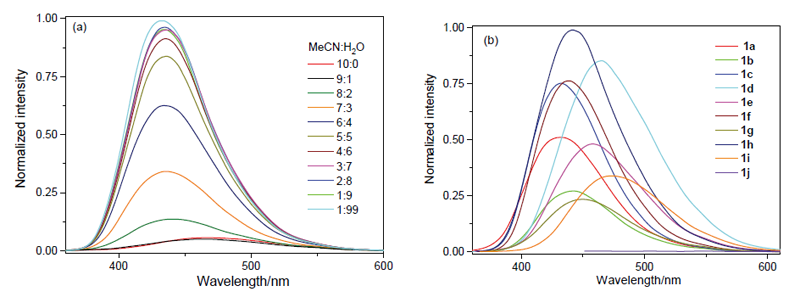
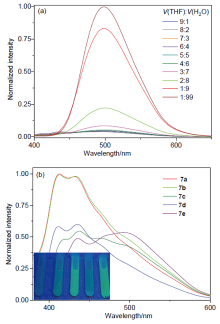

| [1] |
Kwok, R. T. K.; Leung, C. W. T.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2015, 44, 4228.
doi: 10.1039/C4CS00325J |
| [2] |
Ding, D.; Li, K.; Liu, B.; Tang, B. Z. Acc. Chem. Res. 2013, 46, 2441.
doi: 10.1021/ar3003464 |
| [3] |
Luo, J.; Xie, Z.; Lam, J. W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H. S.; Zhan, X.; Liu, Y.; Zhu, D.; Tang, B. Z. Chem. Commun. 2001, 18, 1740.
|
| [4] |
Liu, M.; Gao, P.; Wan, Q.; Deng, F.; Wei, Y.; Zhang, X. Macromol. Rapid Commun. 2017, 38, 1600575.
doi: 10.1002/marc.201600575 |
| [5] |
Wan, Q.; Wang, K.; He, C.; Liu, M.; Zeng, G.; Huang, H.; Deng, F.; Zhang, X.; Wei, Y. Polym. Chem. UK 2015, 6, 8214.
doi: 10.1039/C5PY01513H |
| [6] |
Qin, W. J.; Lam, W.; Yang, Z.; Chen, S.; Liang, W.; Zhao, G.; Kwok, H. S.; Tang, B. Z. Chem. Commun. 2015, 51, 7321.
doi: 10.1039/C5CC01690H |
| [7] |
Li, J.; , Jiang, Cheng, Y. J.; Zhang, Y.; Su, H.; Lam, J. W.; Sung, H. H.; Wong, K. S.; Kwok, H. S.; Tang, B. Z. Phys. Chem. 2015, 17, 1134.
|
| [8] |
Liang, J.; Tang, B. Z.; Liu, B. Chem. Soc. Rev. 2015, 44, 2798.
doi: 10.1039/c4cs00444b pmid: 25686761 |
| [9] |
Singh, V. R.; Malegaonkar, J. N.; Bhosale, S. V.; Singh, P. K. Org. Biomol. Chem. 2020, 18, 8414.
doi: 10.1039/D0OB01661F |
| [10] |
Feng, H. T.; Yuan, Y. X.; Xiong, J. B.; Zheng, Y. S.; Tang, B. Z. Chem. Soc. Rev. 2018, 47, 7452.
doi: 10.1039/C8CS00444G |
| [11] |
Sun, N.; Su, K.; Zhou, Z.; Yu, Y.; Tian, X.; Wang, D.; Zhao, X.; Zhou, H.; Chen, C. ACS Appl. Mater. Interfaces 2018, 10, 16105.
doi: 10.1021/acsami.8b01624 |
| [12] |
La, D. D.; Bhosale, S. V.; Jones, L. A.; Bhosale, S. V. ACS Appl. Mater. Inter. 2017, 10, 12189.
doi: 10.1021/acsami.7b12320 |
| [13] |
Niu, G.; Zheng, X.; Zhao, Z.; Zhang, H.; Wang, J.; He, X.; Tang, B. Z. J. Am. Chem. Soc. 2019, 141, 15111.
doi: 10.1021/jacs.9b06196 |
| [14] |
Jiang, B.; Zhu, W.-H. Sci. China Chem. 2019, 62, 1549.
doi: 10.1007/s11426-019-9630-4 |
| [15] |
Nie, H.; Hu, K.; Cai, Y.; Peng, Q.; Zhao, Z.; Hu, R.; Chen, J.; Su, S.-J.; Qin, A.; Tang, B. Z. Mater. Chem. Front. 2017, 1, 1125
doi: 10.1039/C6QM00343E |
| [16] |
Liu, X.; Li, M.; Liu, M.; Yang, Q.; Chen, Y. Chem. Eur. J. 2018, 24, 13197.
doi: 10.1002/chem.201801507 |
| [17] |
Tong, H.; Hong, Y.; Dong, Y.; Ren, Y.; Häussler, M.; Lam, J. W. Y.; Wong, K. S.; Tang, B. Z. J. Phys. Chem. B 2007, 111, 2000.
doi: 10.1021/jp067374k |
| [18] |
Ding, W.; Cheng, B.; Wang, M.; Dou, Q.; Li, S.; Zhang, P.; Luo, Q. Chin. J. Org. Chem. 2022, 42, 363. (in Chinese)
|
|
( 丁伟, 程勃雯, 王萌, 窦清玉, 李思颖, 张鹏, 罗千福, 有机化学 2022, 42, 363.)
doi: 10.6023/cjoc202108046 |
|
| [19] |
Wang, X.; Ding, G.; Duan, Y.; Zhu, Y.; Zhu, G.; Wang, M.; Li, X.; Zhang, Y.; Qin, X.; Hung, C.-H. Talanta 2020, 217, 121029.
doi: 10.1016/j.talanta.2020.121029 |
| [20] |
Zhao, D.; Han, H. H.; Zhu, L.; Xu, F. Z.; Ma, X. Y.; Li, J.; James, T. D.; Zang, Y.; He, X. P.; Wang, C. ACS Appl. Bio Mater. 2021, 4, 7016.
doi: 10.1021/acsabm.1c00673 pmid: 35006934 |
| [21] |
Tang, F.; Liu, J. Y.; Wu, C. Y.; Liang, Y. X.; Lu, Z. L.; Ding, A. X.; Xu, M. D. ACS Appl. Mater. Interfaces 2021, 13, 23384.
doi: 10.1021/acsami.1c02600 |
| [22] |
Zhang, J.; Chen, Q.; Fan, Y.; Qiu, H.; Ni, Z.; Li, Y.; Yin, S. Dyes Pigm. 2021, 193, 109500.
doi: 10.1016/j.dyepig.2021.109500 |
| [23] |
Tan, G.; Maisuls, I.; Strieth-Kalthoff, F.; Zhang, X.; Daniliuc, C.; Strassert, C. A.; Glorius, F. Adv. Sci. 2021, 8, 2101814.
|
| [24] |
Liu, W.; Wang, Y.; Ge, G.; Ma, L.; Ren, L.; Zhang, Y. Dyes Pigm. 2019, 171, 107704.
doi: 10.1016/j.dyepig.2019.107704 |
| [25] |
Zhao, Y.-H..; Li, Y.; Long, Y.; Zhou, Z.; Tang, Z.; Deng, K.; Zhang, S. Tetrahedron Lett. 2017, 58, 1351.
doi: 10.1016/j.tetlet.2017.02.066 |
| [26] |
Ma, F.; Zhao, G.; Zheng, Y.; He, F.; Hasrat, K.; Qi, Z. ACS Appl. Mater. Inter. 2019, 12, 1179.
doi: 10.1021/acsami.9b17545 |
| [27] |
Cheng, H.-B.; Li, Y.; Tang, B. Z.; Yoon, J. Chem. Soc. Rev. 2020, 49, 21.
doi: 10.1039/C9CS00326F |
| [28] |
Chen, M.; Qin, A.; Lam, J. W. Y.; Tang, B. Z. Coord. Chem. Rev. 2020, 422, 213472.
doi: 10.1016/j.ccr.2020.213472 |
| [29] |
Feng, H.-T.; Lam, J. W. Y.; Tang, B.Z. Coord. Chem. Rev. 2020, 406, 213142.
doi: 10.1016/j.ccr.2019.213142 |
| [30] |
Gao, M.; Tang, B. Z. Coord. Chem. Rev. 2020, 402, 213076.
doi: 10.1016/j.ccr.2019.213076 |
| [31] |
Wen, Y.; Xie, Z.; Shi, T.; Chu, Y.; Zhou, R.; Tao, Y.; Liang, H.; Qiu, H.; Zhao, Y. Chin. J. Org. Chem. 2022, 42, 1463. (in Chinese)
doi: 10.6023/cjoc202111013 |
|
( 文乙评, 解正峰, 史天柱, 褚义成, 周荣贵, 陶奕杉, 梁焕敏, 邱海燕, 赵云辉, 有机化学 2022, 42, 1463.)
doi: 10.6023/cjoc202111013 |
|
| [32] |
Huang, Y.-Y.; Wang, F.-X.; Mu, S.-Y.; Sun, X.; Li, Q.-Z.; Xie, C.-Z.; Liu, H.-B. Spectrochim. Acta A 2020, 243, 118754.
doi: 10.1016/j.saa.2020.118754 |
| [33] |
Zhao, Y. H.; Yu, Y.; Guo, T.; Zhou, Z.; Tang, Z.; Tian, L. Dyes Pigm. 2020, 181, 108547.
doi: 10.1016/j.dyepig.2020.108547 |
| [34] |
Tang, Y.; Yu, Y.; Wei, X.; Yang, J.; Zhu, Y.; Zhao, Y. H.; Tang, Z.; Zhou, Z.; Li, X.; Yu, X. Tetrahedron Lett. 2019, 60, 151187.
doi: 10.1016/j.tetlet.2019.151187 |
| [35] |
Zhao, Y. H.; Li, Y.; Guo, T.; Tang, Z.; Deng, K.; Zhao, G. Synth. Commun. 2016, 46, 355.
doi: 10.1080/00397911.2015.1137944 |
| [36] |
Zhao, Y. H.; Luo, Y.; Wang, H.; Guo, T.; Zhou, H.; Tan, H.; Zhou, Z.; Long, Y.; Tang, Z. ChemistrySelect 2018, 3, 1521.
doi: 10.1002/slct.201702603 |
| [37] |
Zhao, Y.; Luo, M., Li, Y.; Liu, X.; Tang, Z.; Deng, K.; Zhao, G. Chin. J. Chem. 2016, 34, 857.
doi: 10.1002/cjoc.201600277 |
| [38] |
Zhao, Y. H.; Li, Y.; Luo, Y.; Tang, Z.; Deng, K. Synlett 2016, 27, 2597.
doi: 10.1055/s-0035-1562609 |
| [39] |
Zhao, Y. H.; Luo, Y.; Zhu, Y.; Wang, H.; Zhou, H.; Tan, H.; Zhou, Z.; Ma, Y.; Xie, W.; Tang, Z. Synlett 2018, 29, 773.
doi: 10.1055/s-0036-1591743 |
| [40] |
Yang, J.; Wei, X.; Yu, Y.; Zhu, Y.; Zhao, Y. H.; Xie, W.; Zhao, L. Tetrahedron Lett. 2020, 61, 151454.
doi: 10.1016/j.tetlet.2019.151454 |
| [41] |
Zhao, Y.-H.; Yu, Y.; Hu, D.; Zhao, L.; Xie, W.; Zhou, Z. Asian J. Org. Chem. 2020, 9, 953.
doi: 10.1002/ajoc.202000202 |
| [42] |
Zhu, Y.; Yu, Y.; Zhao, Y.-H.; Tang, Z.; Tian, L. Russ. J. Gen. Chem. 2020, 90, 1518.
doi: 10.1134/S1070363220080204 |
| [1] | Penghui Li, Qingyang Xie, Fuxian Wan, Yuanhong Zhang, Lin Jiang. Synthesis and Fungicidal Activity of Novel Substituted Pyrimidine-5-carboxamides Bearing Cyclopropyl Moiety [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 650-656. |
| [2] | Fakai Zou, Nengzhong Wang, Hui Yao, Hui Wang, Mingguo Liu, Nianyu Huang. Regio- and Stereo-selective Synthesis of 1β-/3R-Aryl Thiosugar [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 593-604. |
| [3] | Simin Wu, Jiaxin Tang, Yujia Zhou, Xuetao Xu, Haoxing Zhang, Shaohua Wang. α-Glucosidase Inhibition Research of Derivatives Based on 2β-Acetoxyferruginol Scaffold Excluding Acetic Acid Group [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 613-621. |
| [4] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [5] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [6] | Weiqing Yang, Yanbing Ge, Yuanyuan Chen, Ping Liu, Haiyan Fu, Menglin Ma. Design and Synthesis of Fluorescent 1,8-Napthalimide Derivatives and Their Identification of Cysteine [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 180-194. |
| [7] | Qianfan Zhao, Yongzheng Chen, Shiming Zhang. Application and Mechanism Study of Carbon-Based Metal-Free Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 137-147. |
| [8] | Shan Chen, Zhilin Chen, Qiong Hu, Yanshuang Meng, Yue Huang, Pingfang Tao, Liru Lu, Guobao Huang. Recognition of Bis-thiourea Tweezers to Neutral Molecules in Non-Polar Solvent [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 277-281. |
| [9] | Huakun Wang, Xiaolong Ren, Yining Xuan. Study of the Halide Salt Catalyzed [3+2] Cycloaddition of α,β-Epoxy Carboxylate with Isocyanate [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 251-258. |
| [10] | Yukun Jin, Baoyi Ren, Fushun Liang. Visible Light-Mediated Selective C—F Bond Cleavage of Trifluoromethyl Groups and Its Application in Synthesizing gem-Difluoro-Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 85-110. |
| [11] | Cuiyun Ma, Hailan Luo, Fuhua Zhang, Dan Guo, Shuxing Chen, Fei Wang. Green Biosynthesis, Photophysical Properties and Application of 3-Pyrrolyl BODIPY [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 216-223. |
| [12] | Bozhen Wang, Jie Zhang, Chunhui Nian, Mingming Jin, Miaomiao Kong, Wulan Li, Wenfei He, Jianzhang Wu. Synthesis and Antitumor Activity of 3,4-Dichlorophenyl Amides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 232-241. |
| [13] | Shihang Yu, Jiawei Liu, Biyu An, Qinghua Bian, Min Wang, Jiangchun Zhong. Asymmetric Synthesis of the Contact Sex Pheromone of Neoclytus acuminatus acuminatus (Fabricius) [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 301-308. |
| [14] | Yang Li, Jinding Yuan, Di Zhao. Deep Eutectic Solvent of 1,3-Dimethylurea/L-(+)-Tartaric Acid for the Green Synthesis of (E)-2-Styrylquinoline-3-carboxylic Acid Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3268-3276. |
| [15] | Dandan Sui, Nannan Cen, Ruoqu Gong, Yang Chen, Wenbo Chen. Supporting-Electrolyte-Free Electrochemical Synthesis of Trifluoromethylated Oxindoles in Continuous Flow [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3239-3245. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||