Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (10): 3322-3334.DOI: 10.6023/cjoc202205037 Previous Articles Next Articles
Special Issue: 不对称催化专辑
REVIEWS
收稿日期:2022-05-22
修回日期:2022-06-25
发布日期:2022-11-02
通讯作者:
王春江
基金资助:
Huachao Liua, Chong Shena, Xin Changa, Chunjiang Wanga,b( )
)
Received:2022-05-22
Revised:2022-06-25
Published:2022-11-02
Contact:
Chunjiang Wang
Supported by:Share
Huachao Liu, Chong Shen, Xin Chang, Chunjiang Wang. Recent Advances in Catalytic Asymmetric 1,3-Dipolar Cycloaddition Reactions with Kinetic Resolution[J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3322-3334.
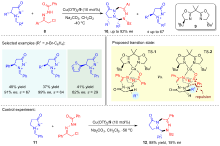
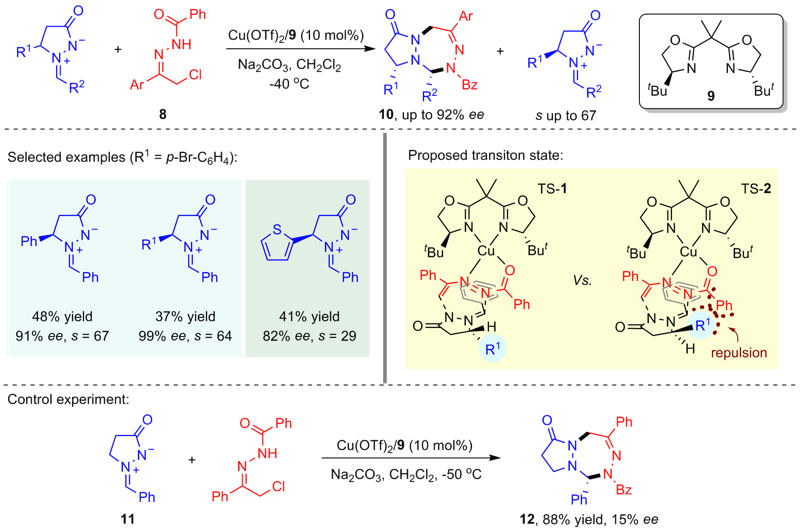
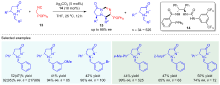
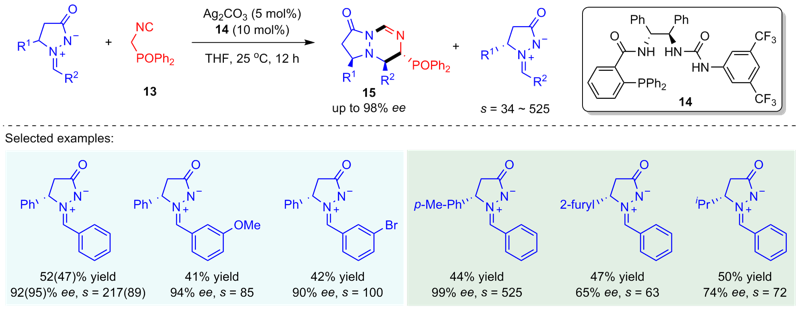

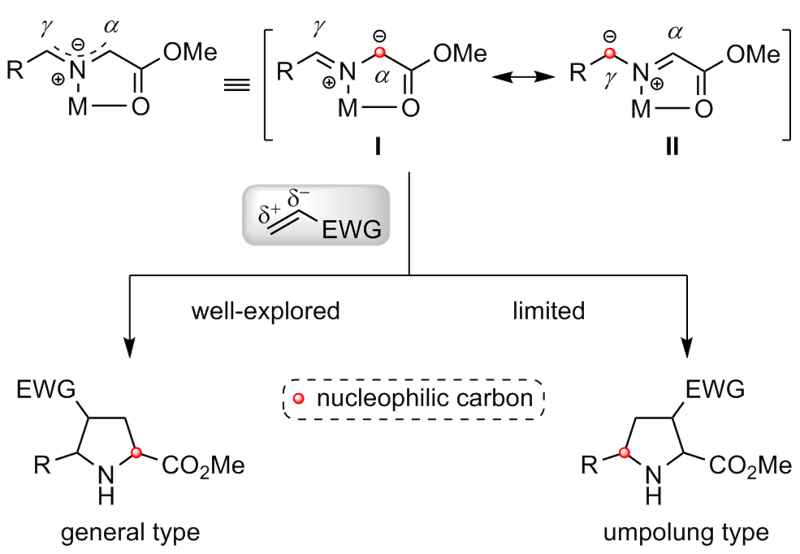
| [1] |
(a) Kagan, H. B.; Fiaud, J. C. Top. Stereochem. 1988, 18, 249.
|
|
(b) Keith, J. M.; Larrow, J. F.; Jacobsen, E. N. Adv. Synth. Catal. 2001, 343, 5.
|
|
|
(c) Robinson, D. E. J. E.; Bull, S. D. Tetrahedron: Asymmetry 2003, 14, 1407.
|
|
|
(d) Vedejs, E.; Jure, M. Angew. Chem., Int. Ed. 2005, 44, 3974.
doi: 10.1002/anie.200460842 |
|
|
(e) Pellissier, H. Adv. Synth. Catal. 2011, 353, 1613.
doi: 10.1002/adsc.201100111 |
|
|
(f) Ding, B.; Xue, Q.; Jia, S.; Cheng, H.-G.; Zhou, Q. Synthesis 2022, 54, 1721.
doi: 10.1055/a-1712-0912 |
|
|
(g) Chang, X.; Che, C.; Wang, Z.-F.; Wang, C.-J. CCS Chem. 2021, 3, 1484.
|
|
|
(h) Wang, S.; Cheng, Z.; Xu, Y.; Yang, L.; Wang, J.-B.; Tian, Z.; Qu, X. Green. Synth. Catal. 2020, 1, 60.
|
|
| [2] |
Pasteur, M. L. C. R. Hebd. Seances Acad. Sci. 1858, 46, 615.
|
| [3] |
For earlier reviews on 1,3-dipolar cycloadditions, see: (a) Huisgen, R. Angew. Chem., Int. Ed. 1963, 2, 565.
pmid: 18613728 |
|
(b) Padwa, A.; Pearson, W. H. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, Wiley-VCH, New York, 2002.
pmid: 18613728 |
|
|
(c) Gothelf, K. V.; Jørgensen, K. A. Chem. Rev. 1998, 98, 863.
pmid: 18613728 |
|
|
(d) Stanley, L.M.; Sibi, M. P. Chem. Rev. 2008, 108, 2887.
doi: 10.1021/cr078371m pmid: 18613728 |
|
| [4] |
For recent reviews on 1,3-dipolar cycloadditions, see: (a) Adrio, J.; Carretero, J. C. Chem. Commun. 2014, 50, 12434.
doi: 10.1039/C4CC04381B pmid: 25961125 |
|
(b) Hashimoto, T.; Maruoka, K. Chem. Rev. 2015, 115, 5366.
doi: 10.1021/cr5007182 pmid: 25961125 |
|
|
(c) Adrio, J.; Carretero, J. C. Chem. Commun. 2019, 55, 11979.
doi: 10.1039/C9CC05238K pmid: 25961125 |
|
|
(d) Wei, L.; Chang, X.; Wang, C.-J. Acc. Chem. Res. 2020, 53, 1084.
doi: 10.1021/acs.accounts.0c00113 pmid: 25961125 |
|
|
(e) Tang, Q.; Zhang, K. Chin. J. Chem. 2021, 39, 3093.
doi: 10.1002/cjoc.202100305 pmid: 25961125 |
|
|
(f) Wang, L.; Zhou, J.; Tang, Y. Chin. J. Chem. 2018, 36, 1123.
doi: 10.1002/cjoc.201800373 pmid: 25961125 |
|
| [5] |
(a) Breugst, M.; Reissig, H.-U. Angew. Chem., Int. Ed. 2020, 59, 12293.
doi: 10.1002/anie.202003115 |
|
(b) Heusgen, R. J. Org. Chem. 1968, 33, 2291.
doi: 10.1021/jo01270a024 |
|
|
(c) Firestone, R. A. J. Org. Chem. 1968, 33, 2285.
doi: 10.1021/jo01270a023 |
|
|
(d) Poppinger, D. J. Am. Chem. Soc. 1975, 97, 7486.
doi: 10.1021/ja00859a017 |
|
|
(e) Huisgen, R. Chemistry and Biological Applications of Oxygen- and Sulfur-Containing Heterocycles, Eds.: Kartsev, V. G., IBX Press, Moscow, 2003, p. 83.
|
|
| [6] |
For a review, see: Cardona, F.; Goti, A.; Brandi, A. Eur. J. Org. Chem. 2001, 2999.
|
| [7] |
For reviews on the studies of azomethine imines, see: (a) Schantl, J. G. Sci. Synth. 2004, 27, 731.
|
|
(b) Grashey, R. In 1,3-Dipolar Cycloaddition Chemistry, Vol. 1, Eds.: Padwa, A., Wiley, New York, 1984, p. 733.
|
|
| [8] |
For reviews on the chemistry and biology of pyrazolidinones, see: (a) Claramunt, R. M.; Elguero, J. Org. Prep. Proced. Int. 1991, 23, 273.
doi: 10.1080/00304949109458208 |
|
(b) Konaklieva, M. I.; Plotkin, B. J. Curr. Med. Chem.: Anti-Infect. Agents 2003, 2, 287, and references cited therein.
|
|
| [9] |
(a) Bongers, A.; Moon, P. J.; Beauchemin, A. M. Angew. Chem., Int. Ed. 2015, 54, 15516.
doi: 10.1002/anie.201507548 |
|
(b) Wang, H.-Y.; Zheng, C.-W.; Chai, Z.; Zhang, J.-X.; Zhao, G. Nat. Commun. 2016, 7, 12720.
doi: 10.1038/ncomms12720 |
|
| [10] |
(a) Shintani, R.; Fu, G. C. J. Am. Chem. Soc. 2003, 125, 10778.
pmid: 12952444 |
|
(b) Suarez, A.; Downey, C. W.; Fu, G. C. J. Am. Chem. Soc. 2005, 127, 11244.
doi: 10.1021/ja052876h pmid: 12952444 |
|
| [11] |
Wang, M.; Huang, Z.; Xu, J.; Chi, Y. R. J. Am. Chem. Soc. 2014, 136, 1214.
doi: 10.1021/ja411110f |
| [12] |
Wei, L. Wang, Z.-F.; Yao, L.; Qiu, G.; Tao, H.-Y.; Li, H.; Wang, C.-J. Adv. Synth. Catal. 2016, 358, 3955.
doi: 10.1002/adsc.201600961 |
| [13] |
(a) Evans, D. A.; Rovis, T.; Kozlowski, M. C.; Tedrow, J. S. J. Am. Chem. Soc. 1999, 121, 1994.
doi: 10.1021/ja983864h |
|
(b) Evans, D. A.; Miller, S. J.; Lectka, T.; von Matt, P. J. Am. Chem. Soc. 1999, 121, 7559.
doi: 10.1021/ja991190k |
|
| [14] |
Tao, L.-F; Zhang, S.; Huang, F.; Wang, W.-T.; Luo, Z.-H.; Qin, L.; Liao, J.-Y. Angew. Chem. Int. Ed. 2022, 61, e202202679.
|
| [15] |
(a) Xie, J.; Yoshida, K.; Takasu, K.; Takemoto, Y. Tetrahedron Lett. 2008, 49, 6910.
doi: 10.1016/j.tetlet.2008.09.113 |
|
(b) Xie, J.-W.; Fan, L.-P.; Su, H.; Li, X.-S.; Xu, D.-C. Org. Biomol. Chem. 2010, 8, 2117.
doi: 10.1039/b922668k |
|
| [16] |
(a) Zeni, G.; Larock, R. C. Chem. Rev. 2004, 104, 2285.
doi: 10.1021/cr020085h |
|
(b) Bonsignore, L.; Loy, G.; Secci, D.; Calignano, A. Eur. J. Med. Chem. 1993, 28, 517.
doi: 10.1016/0223-5234(93)90020-F |
|
|
(c) Machlin, L. J. α-Tocopherol, Eds.: Dekker, M., New York, 1980, p. 345.
|
|
| [17] |
Yu, J.; Chen, W.-J.; Gong, L.-Z. Org. Lett. 2010, 12, 4050.
doi: 10.1021/ol101544c |
| [18] |
(a) Krause, N. Modern Allene Chemistry, Eds.: Hashmi, A. S. K., Wiley-VCH, Weinheim, 2004.
pmid: 24676356 |
|
(b) Hoffmann-Roder, A.; Krause, N. Angew. Chem., Int. Ed. 2004, 43, 1196.
doi: 10.1002/anie.200300628 pmid: 24676356 |
|
|
(c) Alcaide, B.; Almendros, P. Chem. Soc. Rev. 2014, 43, 2886, and references cited therein.
doi: 10.1039/c4cs90020k pmid: 24676356 |
|
| [19] |
Takayama, H.; Jia, Z.-J.; Kremer, L.; Bauer, J. O.; Strohmann, C.; Ziegler, S.; Antonchick, A. P.; Waldmann, H. Angew. Chem., Int. Ed. 2013, 52, 12404.
doi: 10.1002/anie.201306948 |
| [20] |
Xu, H.; Golz, C.; Strohmann, C.; Antonchick, A. P.; Waldmann, H. Angew. Chem., Int. Ed. 2016, 55, 7761.
doi: 10.1002/anie.201602084 |
| [21] |
Yuan, Y.; Zheng, Z.-J.; Li, L.; Bai, X.-F.; Xu, Z.; Cui, Y.-M.; Cao, J.; Yang, K.-F.; Xu, L.-W. Adv. Synth. Catal. 2018, 360, 3002.
doi: 10.1002/adsc.201800220 |
| [22] |
(a) Liu, H.-C.; Liu, K.; Xue, Z.-Y.; He, Z.-L.; Wang, C.-J. Org. Lett. 2015, 17, 5440.
doi: 10.1021/acs.orglett.5b02810 pmid: 29846076 |
|
(b) Liu, H.-C.; Wei, L.; Huang, R.; Tao, H.-Y.; Cong, H.; Wang, C.-J. Org. Lett. 2018, 20, 3482.
doi: 10.1021/acs.orglett.8b01254 pmid: 29846076 |
|
|
(a) Aikawa, K.; Okamoto, T.; Mikami, K. J. Am. Chem. Soc. 2012, 134, 10329.
doi: 10.1021/ja3032345 pmid: 29846076 |
|
|
(b) Hirose, T.; Sunazuka, T.; Yamamoto, D.; Kojima, N.; Shirahata, T.; Harigaya, Y.; Kuwajima, I.; Ōmura, S. Tetrahedron 2005, 61, 6015.
doi: 10.1016/j.tet.2005.04.056 pmid: 29846076 |
|
| [23] |
(a) Liu, X.; Zhang, L.-N.; Leng, Y. Acta Pharmacol. Sin. 2012, 33, 1013.
doi: 10.1038/aps.2012.75 pmid: 20092908 |
|
(b) Zhao, C.; Sun, M.-H.; Cowart, M. D. J. Med. Chem. 2008, 51, 5423.
doi: 10.1021/jm8003625 pmid: 20092908 |
|
|
(c) Doebele, R. C.; Oton, A. B.; Peled, N.; Camidge, D. R.; Bunn, P. A., Jr. Lung Cancer 2010, 69, 1.
doi: 10.1016/j.lungcan.2009.12.009 pmid: 20092908 |
|
| [24] |
(a) Barr, D. A.; Grigg, R.; Sridharan, V. Tetrahedron Lett. 1989, 30, 4727.
doi: 10.1016/S0040-4039(01)80786-3 pmid: 19736987 |
|
(b) Kanemasa, S.; Uchida, O.; Wada, E.; Yamamoto, H. Chem. Lett. 1990, 19, 105.
doi: 10.1246/cl.1990.105 pmid: 19736987 |
|
|
(c) Chen, X.-H.; Wei, Q.; Luo, S.-W.; Xiao, H.; Gong, L.-Z. J. Am. Chem. Soc. 2009, 131, 13819.
doi: 10.1021/ja905302f pmid: 19736987 |
|
|
(d) Feng, B.; Lu, L.-Q.; Chen, J.-R.; Feng, G.; He, B.-Q.; Lu, B.; Xiao, W.-J. Angew. Chem., Int. Ed. 2018, 57, 5888.
doi: 10.1002/anie.201802492 pmid: 19736987 |
|
|
(e) Xu, S.; Zhang, Z.-M.; Xu, B.; Liu, B.; Liu, Y.; Zhang, J. J. Am. Chem. Soc. 2018, 140, 2272.
doi: 10.1021/jacs.7b12137 pmid: 19736987 |
|
|
(f) Stohler, R.; Wahl, F.; Pfaltz, A. Synthesis 2005, 1431.
pmid: 19736987 |
|
| [25] |
Shen, C.; Yang, Y.; Wei, L.; Dong, W.-W.; Chung, L. W.; Wang, C.-J. iScience 2019, 11, 146.
doi: 10.1016/j.isci.2018.12.010 |
| [26] |
Wang, M.; Wang, C.-J.; Lin, Z. Organometallics 2012, 31, 7870.
doi: 10.1021/om300435s |
| [27] |
Krotz, A.; Helmchen, G. Liebigs Ann. Chem. 1994, 6, 601.
|
| [28] |
Chang, X.; Sun, X.-S.; Che, C.; Hu, Y.-Z.; Tao, H.-Y.; Wang, C.-J. Org. Lett. 2019, 21, 1191.
doi: 10.1021/acs.orglett.9b00136 pmid: 30707591 |
| [29] |
Vázquez-Romero, A.; Rodríguez, J.; Lledó, A.; Verdaguer, X.; Riera, A. Org. Lett. 2008, 10, 4509.
doi: 10.1021/ol8017352 pmid: 18798645 |
| [30] |
Deng, H.; Liu, T.-T.; Ding, Z.-D.; Yang, W.-L.; Luo, X.; Deng, W.-P. Org. Chem. Front. 2020, 7, 3247.
doi: 10.1039/D0QO00789G |
| [1] | Shuang Yang, Xinqiang Fang. Kinetic Resolutions Enabled by N-Heterocyclic Carbene Catalysis: An Update [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 448-480. |
| [2] | Zuliang Chen, Yingjing Wei, Junliang Zhang. Recent Advances in Cycloaddition Reactions of Donor-Acceptor Aziridines via Carbon-Carbon Bond Cleavage [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3078-3088. |
| [3] | Xiaoke Zhang, Xiangru Zheng, Chaoyong Wang. Strategy to Construct Functionalized Tetrazepine Derivatives via [4+3] Annulation Reaction of Azomethine Imine with Azadiene Precursor [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3180-3187. |
| [4] | Huijuan Hu, Qiaoli Yan, Xiaogang Lu, Qifan Yang, Chengxin Pei, Hongmei Wang, Runli Gao. Kinetic Resolution of Racemic P-Chiral α-Hydroxymethylphos-phonates Catalyzed by Lipase from Porcine Pancreas [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2815-2825. |
| [5] | Yangyang Chu, Zhaobin Han, Kuiling Ding. Progresses in the Application of Kinetic Resolution in Transition Metal Catalyzed Asymmetric (Transfer) Hydrogenation [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1934-1951. |
| [6] | Cheng Luo, Yanli Yin, Zhiyong Jiang. Recent Advances in Asymmetric Synthesis of P-Chiral Phosphine Oxides [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 1963-1976. |
| [7] | Yuliang Chen, Fengkai He, Siyun Wang, Dingcheng Jia, Yaqun Liu, Yiyong Huang. Kinetic Resolution of Aldehydes Bearing an All-Carbon Quaternary Stereocenter at the α-Position by the Antilla Allylboration [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4294-4302. |
| [8] | Yunrong Chen, Wei Liu, Xiaoyu Yang. Recent Advances in Kinetic Resolution of Tertiary Alcohols [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 679-697. |
| [9] | Lingjie Fan, Tao Zhou, Xu Yang, Mengxue Jiang, Xinquan Hu, Bingfeng Shi. Pd(II)-Catalyzed Enantioselective C—H Olefination of 2-(Arylsulfinyl)pyridines through Kinetic Resolution [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3405-3418. |
| [10] | Hui Li, Liang Yin. Research Progress on Catalytic Asymmetric Synthesis of P-Chiral Compounds [J]. Chinese Journal of Organic Chemistry, 2022, 42(10): 3183-3200. |
| [11] | Tang Liang, Li Xuewei, Xie Fang, Zhang Wanbin. Catalytic Kinetic Resolution of Amines and Their Derivatives by Non-acylation Reaction [J]. Chinese Journal of Organic Chemistry, 2020, 40(3): 575-588. |
| [12] | Zhang Qiying, Zhang Yiming, Hao Erjun, Bai Juan, Qu Guirong, Guo Haiming. Asymmetric Transfer Hydrogenation via Dynamic Kinetic Resolution for the Construction of Carbocyclic N3-Purine Nucleosides [J]. Chinese Journal of Organic Chemistry, 2020, 40(2): 376-383. |
| [13] | Hua Tingbi, Yang Qingqing, Xiao Wengjing. Recent Developments of Reactions with C,N-Cyclic Azomethine Imines [J]. Chinese Journal of Organic Chemistry, 2020, 40(11): 3559-3595. |
| [14] | Yue Guizhou, Liu Bo. Research Progress on [3+n] (n≥3) Cycloaddition of 1,3-Diploes [J]. Chinese Journal of Organic Chemistry, 2020, 40(10): 3132-3153. |
| [15] | Wang Cai, Zhou Feng, Zhou Jian. Recent Advances in the Enantioselective Copper(I)-Catalyzed Azide-Alkyne Cycloaddition Reaction [J]. Chinese Journal of Organic Chemistry, 2020, 40(10): 3065-3077. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||