Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (11): 3824-3834.DOI: 10.6023/cjoc202205048 Previous Articles Next Articles
ARTICLES
高潮a,b, 张玉桐a,b, 池玲玲a,b, 王浩a,b, 马家婕a,b, 毕梦鑫a,b, 戴洪林a,b, 司晓杰a,b, 刘丽敏a,b, 张洋a,b, 郑甲信a,b, 可钰a,b,c,d,*( ), 刘宏民a,b,c,d,*(
), 刘宏民a,b,c,d,*( ), 张秋荣a,b,c,d,*(
), 张秋荣a,b,c,d,*( )
)
收稿日期:2022-05-28
修回日期:2022-06-27
发布日期:2022-07-13
通讯作者:
可钰, 刘宏民, 张秋荣
基金资助:
Chao Gaoa,b, Yutong Zhanga,b, Lingling Chia,b, Hao Wanga,b, Jiajie Maa,b, Mengxin Bia,b, Honglin Daia,b, Xiaojie Sia,b, Limin Liua,b, Yang Zhanga,b, Jiaxin Zhenga,b, Yu Kea,b,c,d( ), Hongmin Liua,b,c,d(
), Hongmin Liua,b,c,d( ), Qiurong Zhanga,b,c,d(
), Qiurong Zhanga,b,c,d( )
)
Received:2022-05-28
Revised:2022-06-27
Published:2022-07-13
Contact:
Yu Ke, Hongmin Liu, Qiurong Zhang
Supported by:Share
Chao Gao, Yutong Zhang, Lingling Chi, Hao Wang, Jiajie Ma, Mengxin Bi, Honglin Dai, Xiaojie Si, Limin Liu, Yang Zhang, Jiaxin Zheng, Yu Ke, Hongmin Liu, Qiurong Zhang. Synthesis and Antiproliferative Activity Evaluation of Novel 2,4,6-Trisubstituted Pyrimidine Derivatives[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3824-3834.
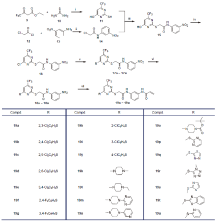
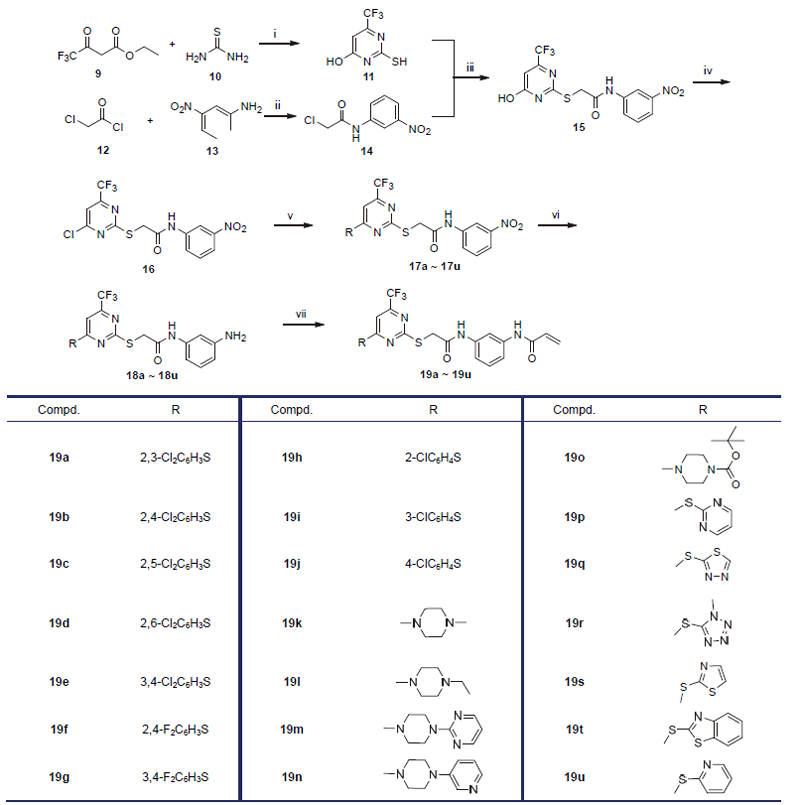
| Compd. | R | IC50a/(μmol•L-1) | |||
|---|---|---|---|---|---|
| PC-3 | MGC-803 | MCF-7 | HGC-27 | ||
| 19a | 2,3-Cl2C6H3S | 11.20±1.05 | 4.24±0.63 | 15.28±1.18 | 13.70±1.14 |
| 19b | 2,4-Cl2C6H3S | 15.17±1.18 | 4.60±0.66 | 14.49±1.16 | 13.49±1.13 |
| 19c | 2,5-Cl2C6H3S | 11.94±1.08 | 4.41±0.67 | 12.85±1.11 | 10.75±1.03 |
| 19d | 2,6-Cl2C6H3S | 12.57±0.96 | 10.05±1.02 | 13.93±1.14 | 12.45±1.09 |
| 19e | 3,4-Cl2C6H3S | 11.65±1.07 | 7.53±0.88 | 16.18±1.21 | 11.16±1.05 |
| 19f | 2,4-F2C6H3S | 24.18±1.38 | 4.29±1.03 | 26.54±1.42 | 22.83±1.36 |
| 19g | 3,4-F2C6H3S | 22.54±1.35 | 4.47±1.09 | 24.16±1.38 | 22.24±1.35 |
| 19h | 2-ClC6H4S | 13.20±1.12 | 4.57±0.87 | 16.96±1.23 | 21.60±1.33 |
| 19i | 3-ClC6H4S | 13.22±1.04 | 3.95±1.22 | 20.10±1.30 | 18.20±1.26 |
| 19j | 4-ClC6H4S | 16.29±1.15 | 6.09±0.78 | 18.71±1.27 | 16.70±1.22 |
| 19k | | 19.23±1.28 | 2.51±0.57 | 22.42±1.35 | 22.75±1.35 |
| 19l | | 12.09±1.08 | 7.64±0.88 | 9.13±0.96 | 10.18±1.01 |
| 19m | | 5.97±0.85 | 15.72±1.16 | 19.27±1.28 | 16.08±1.21 |
| 19n | | 10.21±1.01 | 15.42±1.19 | 13.12±1.12 | 13.77±1.14 |
| 19o | | 11.26±1.06 | 25.71±1.41 | 20.96±1.32 | 17.23±1.24 |
| 19p | | 2.30±0.21 | 3.55±0.55 | 5.46±0.74 | 3.64±0.56 |
| 19q | | 0.87±0.15 | 0.97±0.18 | 1.38±0.14 | 1.17±0.07 |
| 19r | | 0.96±0.11 | 1.36±0.16 | 1.77±0.25 | 1.93±0.29 |
| 19s | | 1.86±0.27 | 2.19±0.34 | 3.57±0.55 | 1.51±0.18 |
| 19t | | 4.15±0.62 | 8.61±0.94 | 2.71±0.43 | 2.81±0.45 |
| 19u | | 8.30±0.92 | 9.66±0.98 | 17.22±1.24 | 8.01±0.90 |
| 5-Fub | — | 6.39±0.71 | 10.64±1.09 | 10.23±0.68 | 7.25±0.72 |
| Compd. | R | IC50a/(μmol•L-1) | |||
|---|---|---|---|---|---|
| PC-3 | MGC-803 | MCF-7 | HGC-27 | ||
| 19a | 2,3-Cl2C6H3S | 11.20±1.05 | 4.24±0.63 | 15.28±1.18 | 13.70±1.14 |
| 19b | 2,4-Cl2C6H3S | 15.17±1.18 | 4.60±0.66 | 14.49±1.16 | 13.49±1.13 |
| 19c | 2,5-Cl2C6H3S | 11.94±1.08 | 4.41±0.67 | 12.85±1.11 | 10.75±1.03 |
| 19d | 2,6-Cl2C6H3S | 12.57±0.96 | 10.05±1.02 | 13.93±1.14 | 12.45±1.09 |
| 19e | 3,4-Cl2C6H3S | 11.65±1.07 | 7.53±0.88 | 16.18±1.21 | 11.16±1.05 |
| 19f | 2,4-F2C6H3S | 24.18±1.38 | 4.29±1.03 | 26.54±1.42 | 22.83±1.36 |
| 19g | 3,4-F2C6H3S | 22.54±1.35 | 4.47±1.09 | 24.16±1.38 | 22.24±1.35 |
| 19h | 2-ClC6H4S | 13.20±1.12 | 4.57±0.87 | 16.96±1.23 | 21.60±1.33 |
| 19i | 3-ClC6H4S | 13.22±1.04 | 3.95±1.22 | 20.10±1.30 | 18.20±1.26 |
| 19j | 4-ClC6H4S | 16.29±1.15 | 6.09±0.78 | 18.71±1.27 | 16.70±1.22 |
| 19k | | 19.23±1.28 | 2.51±0.57 | 22.42±1.35 | 22.75±1.35 |
| 19l | | 12.09±1.08 | 7.64±0.88 | 9.13±0.96 | 10.18±1.01 |
| 19m | | 5.97±0.85 | 15.72±1.16 | 19.27±1.28 | 16.08±1.21 |
| 19n | | 10.21±1.01 | 15.42±1.19 | 13.12±1.12 | 13.77±1.14 |
| 19o | | 11.26±1.06 | 25.71±1.41 | 20.96±1.32 | 17.23±1.24 |
| 19p | | 2.30±0.21 | 3.55±0.55 | 5.46±0.74 | 3.64±0.56 |
| 19q | | 0.87±0.15 | 0.97±0.18 | 1.38±0.14 | 1.17±0.07 |
| 19r | | 0.96±0.11 | 1.36±0.16 | 1.77±0.25 | 1.93±0.29 |
| 19s | | 1.86±0.27 | 2.19±0.34 | 3.57±0.55 | 1.51±0.18 |
| 19t | | 4.15±0.62 | 8.61±0.94 | 2.71±0.43 | 2.81±0.45 |
| 19u | | 8.30±0.92 | 9.66±0.98 | 17.22±1.24 | 8.01±0.90 |
| 5-Fub | — | 6.39±0.71 | 10.64±1.09 | 10.23±0.68 | 7.25±0.72 |

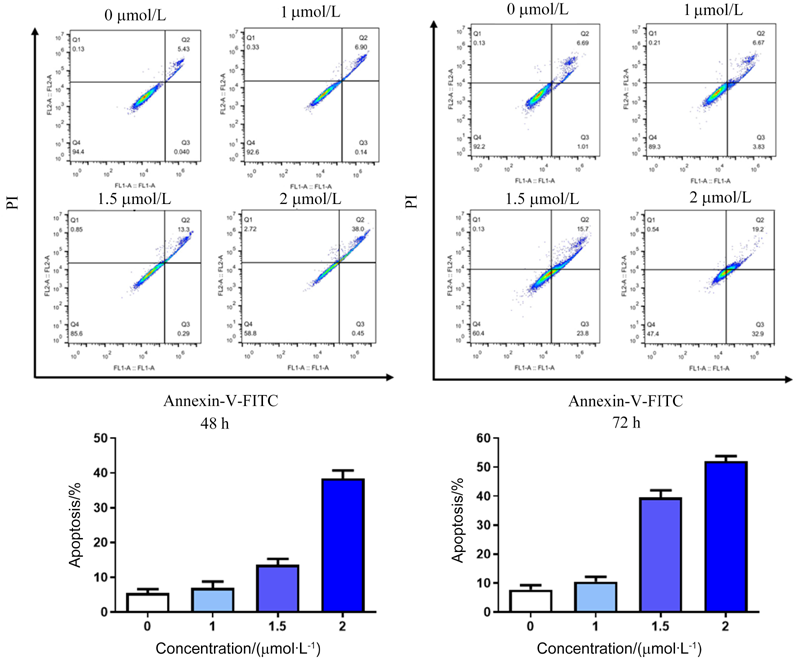


| [1] |
Siegel, R. L.; Miller, K. D.; Fuchs, H. E.; Jemal, A. Ca-Cancer J. Clin. 2022, 72, 7.
doi: 10.3322/caac.21708 |
| [2] |
Hernandez-Lopez, A.; Tellez-Gonzalez, M. A.; Mondragon-Teran, P.; Meneses-Acosta, A. Front Pharmacol. 2021, 12, 720692.
doi: 10.3389/fphar.2021.720692 |
| [3] |
Giri, V. N.; Morgan, T. M.; Morris, D. S.; Berchuck, J. E.; Hyatt, C.; Taplin, M. E. Ca-Cancer J. Clin. 2022, 73, 1.
|
| [4] |
Guo, X.; Yang, N.; Ji, W.; Zhang, H.; Dong, X.; Zhou, Z.; Li, L.; Shen, H. M.; Yao, S. Q.; Huang, W. Adv. Mater. 2021, 33, 2007778.
doi: 10.1002/adma.202007778 |
| [5] |
Gu, X.; Huang, Z.; Ren, Z.; Tang, X.; Xue, R.; Luo, X.; Peng, S.; Peng, H.; Lu, B.; Tian, J.; Zhang, Y. J. Med. Chem. 2017, 60, 928.
doi: 10.1021/acs.jmedchem.6b01075 |
| [6] |
Tian, B.; Liu, Y.; Liu, J. Eur. Polym. J. 2021, 154, 110533.
doi: 10.1016/j.eurpolymj.2021.110533 |
| [7] |
Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Rout, L.; Jena, M.; Efferth, T.; Bhutia, S. K. Semin. Cancer Biol. 2021, 73, 310.
doi: 10.1016/j.semcancer.2020.10.010 |
| [8] |
Passaro, A.; Guerini-Rocco, E.; Pochesci, A.; Vacirca, D.; Spitaleri, G.; Catania, C. M.; Rappa, A.; Barberis, M.; de Marinis, F. Pharmacol. Res. 2017, 117, 406.
doi: 10.1016/j.phrs.2017.01.003 |
| [9] |
Finlay, M. R.; Anderton, M.; Ashton, S.; Ballard, P.; Bethel, P. A.; Box, M. R.; Bradbury, R. H.; Brown, S. J.; Butterworth, S.; Campbell, A.; Chorley, C.; Colclough, N.; Cross, D. A.; Currie, G. S.; Grist, M.; Hassall, L.; Hill, G. B.; James, D.; James, M.; Kemmitt, P.; Klinowska, T.; Lamont, G.; Lamont, S. G.; Martin, N.; McFarland, H. L.; Mellor, M. J.; Orme, J. P.; Perkins, D.; Perkins, P.; Richmond, G.; Smith, P.; Ward, R. A.; Waring, M. J.; Whittaker, D.; Wells, S.; Wrigley, G. L. J. Med. Chem. 2014, 57, 8249.
doi: 10.1021/jm500973a |
| [10] |
Meng, Y.; Li, E.; Zhang, Y.; Liu, S.; Bao, C.; Yang, P.; Zhang, L.; Zhang, D.; Wang, J.; Chen, Y.; Li, N.; Xin, J.; Zhao, P.; Ke, Y.; Zhang, Q.; Liu, H. Chin. J. Org. Chem. 2019, 39, 2541. (in Chinese)
doi: 10.6023/cjoc201903022 |
|
( 孟娅琪, 李二冬, 张洋, 刘栓, 包崇男, 杨鹏, 张路野, 张丹青, 王继宽, 陈雅欣, 栗娜, 辛景超, 赵培荣, 可钰, 张秋荣, 刘宏民, 有机化学 2019, 39, 2541.)
doi: 10.6023/cjoc201903022 |
|
| [11] |
Meanwell, N. A. J. Med. Chem. 2018, 61, 5822.
doi: 10.1021/acs.jmedchem.7b01788 |
| [12] |
Clegg, N. J.; Wongvipat, J.; Joseph, J. D.; Tran, C.; Ouk, S.; Dilhas, A.; Chen, Y.; Grillot, K.; Bischoff, E. D.; Cai, L.; Aparicio, A.; Dorow, S.; Arora, V.; Shao, G.; Qian, J.; Zhao, H.; Yang, G.; Cao, C.; Sensintaffar, J.; Wasielewska, T.; Herbert, M. R.; Bonnefous, C.; Darimont, B.; Scher, H. I.; Smith-Jones, P.; Klang, M.; Smith, N. D.; De Stanchina, E.; Wu, N.; Ouerfelli, O.; Rix, P. J.; Heyman, R. A.; Jung, M. E.; Sawyers, C. L.; Hager, J. H. Cancer Res. 2012, 72, 1494.
|
| [13] |
Stein, E. M.; DiNardo, C. D.; Fathi, A. T.; Pollyea, D. A.; Stone, R. M.; Altman, J. K.; Roboz, G. J.; Patel, M. R.; Collins, R.; Flinn, I. W.; Sekeres, M. A.; Stein, A. S.; Kantarjian, H. M.; Levine, R. L.; Vyas, P.; MacBeth, K. J.; Tosolini, A.; VanOostendorp, J.; Xu, Q.; Gupta, I.; Lila, T.; Risueno, A.; Yen, K. E.; Wu, B.; Attar, E. C.; Tallman, M. S.; de Botton, S. Blood 2019, 133, 676.
doi: 10.1182/blood-2018-08-869008 |
| [14] |
Chen, L.; Zhang, Y.; Liu, J.; Wang, W.; Li, X.; Zhao, L.; Wang, W.; Li, B. Eur. J. Med. Chem. 2017, 138, 689.
doi: S0223-5234(17)30469-5 pmid: 28711703 |
| [15] |
Hou, W.; Ren, Y.; Zhang, Z.; Sun, H.; Ma, Y.; Yan, B. Bioorg. Med. Chem. 2018, 26, 1740.
doi: 10.1016/j.bmc.2018.02.022 |
| [16] |
Patel, H.; Pawara, R.; Ansari, A.; Surana, S. Eur. J. Med. Chem. 2017, 142, 32.
doi: 10.1016/j.ejmech.2017.05.027 |
| [17] |
Liang, X.; Wang, B.; Chen, C.; Wang, A.; Hu, C.; Zou, F.; Yu, K.; Liu, Q.; Li, F.; Hu, Z.; Lu, T.; Wang, J.; Wang, L.; Weisberg, E. L.; Li, L.; Xia, R.; Wang, W.; Ren, T.; Ge, J.; Liu, J.; Liu, Q. J. Med. Chem. 2019, 62, 875.
doi: 10.1021/acs.jmedchem.8b01594 |
| [18] |
Wu, Y.; Wang, B.; Wang, J.; Qi, S.; Zou, F.; Qi, Z.; Liu, F.; Liu, Q.; Chen, C.; Hu, C.; Hu, Z.; Wang, A.; Wang, L.; Wang, W.; Ren, T.; Cai, Y.; Bai, M.; Liu, Q.; Liu, J. J. Med. Chem. 2019, 62, 6083.
doi: 10.1021/acs.jmedchem.9b00280 |
| [19] |
Fu, S.; Chen, X.; Lo, H. W.; Lin, J. Cancer Lett. 2019, 448, 11.
doi: 10.1016/j.canlet.2019.01.026 |
| [20] |
Abdel-Mohsen, H. T.; Conrad, J.; Harms, K.; Nohr, D.; Beifuss, U. RSC Adv. 2017, 7, 17427.
doi: 10.1039/C6RA28102H |
| [21] |
Tala, S. D.; Ou, T.-H.; Lin, Y.-W.; Tala, K. S.; Chao, S.-H.; Wu, M.-H.; Tsai, T.-H.; Kakadiya, R.; Suman, S.; Chen, C.-H.; Lee, T.-C.; Su, T.-L. Eur. J. Med. Chem. 2014, 76, 155.
doi: 10.1016/j.ejmech.2014.02.018 |
| [22] |
Ma, L. Y.; Zheng, Y. C.; Wang, S. Q.; Wang, B.; Wang, Z. R.; Pang, L. P.; Zhang, M.; Wang, J. W.; Ding, L.; Li, J.; Wang, C.; Hu, B.; Liu, Y.; Zhang, X. D.; Wang, J. J.; Wang, Z. J.; Zhao, W.; Liu, H. M. J. Med. Chem. 2015, 58, 1705.
doi: 10.1021/acs.jmedchem.5b00037 pmid: 25610955 |
| [23] |
Ge, Y.; Yang, H.; Wang, C.; Meng, Q.; Li, L.; Sun, H.; Zhen, Y.; Liu, K.; Li, Y.; Ma, X. Bioorg. Med. Chem. 2017, 25, 765.
doi: 10.1016/j.bmc.2016.11.054 |
| [1] | Fakai Zou, Nengzhong Wang, Hui Yao, Hui Wang, Mingguo Liu, Nianyu Huang. Regio- and Stereo-selective Synthesis of 1β-/3R-Aryl Thiosugar [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 593-604. |
| [2] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [3] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [4] | Penghui Li, Qingyang Xie, Fuxian Wan, Yuanhong Zhang, Lin Jiang. Synthesis and Fungicidal Activity of Novel Substituted Pyrimidine-5-carboxamides Bearing Cyclopropyl Moiety [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 650-656. |
| [5] | Weiqing Yang, Yanbing Ge, Yuanyuan Chen, Ping Liu, Haiyan Fu, Menglin Ma. Design and Synthesis of Fluorescent 1,8-Napthalimide Derivatives and Their Identification of Cysteine [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 180-194. |
| [6] | Shihang Yu, Jiawei Liu, Biyu An, Qinghua Bian, Min Wang, Jiangchun Zhong. Asymmetric Synthesis of the Contact Sex Pheromone of Neoclytus acuminatus acuminatus (Fabricius) [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 301-308. |
| [7] | Qianfan Zhao, Yongzheng Chen, Shiming Zhang. Application and Mechanism Study of Carbon-Based Metal-Free Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 137-147. |
| [8] | Shan Chen, Zhilin Chen, Qiong Hu, Yanshuang Meng, Yue Huang, Pingfang Tao, Liru Lu, Guobao Huang. Recognition of Bis-thiourea Tweezers to Neutral Molecules in Non-Polar Solvent [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 277-281. |
| [9] | Huakun Wang, Xiaolong Ren, Yining Xuan. Study of the Halide Salt Catalyzed [3+2] Cycloaddition of α,β-Epoxy Carboxylate with Isocyanate [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 251-258. |
| [10] | Yukun Jin, Baoyi Ren, Fushun Liang. Visible Light-Mediated Selective C—F Bond Cleavage of Trifluoromethyl Groups and Its Application in Synthesizing gem-Difluoro-Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 85-110. |
| [11] | Cuiyun Ma, Hailan Luo, Fuhua Zhang, Dan Guo, Shuxing Chen, Fei Wang. Green Biosynthesis, Photophysical Properties and Application of 3-Pyrrolyl BODIPY [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 216-223. |
| [12] | Bozhen Wang, Jie Zhang, Chunhui Nian, Mingming Jin, Miaomiao Kong, Wulan Li, Wenfei He, Jianzhang Wu. Synthesis and Antitumor Activity of 3,4-Dichlorophenyl Amides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 232-241. |
| [13] | Yang Li, Jinding Yuan, Di Zhao. Deep Eutectic Solvent of 1,3-Dimethylurea/L-(+)-Tartaric Acid for the Green Synthesis of (E)-2-Styrylquinoline-3-carboxylic Acid Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3268-3276. |
| [14] | Dandan Sui, Nannan Cen, Ruoqu Gong, Yang Chen, Wenbo Chen. Supporting-Electrolyte-Free Electrochemical Synthesis of Trifluoromethylated Oxindoles in Continuous Flow [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3239-3245. |
| [15] | Ruixia Cao, Yuping Jia. Synthesis and Biological Activity of Novel Pyrrolo[2,3-d]pyrimidine Derivatives Containing Coumarin [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3304-3311. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||