-
-
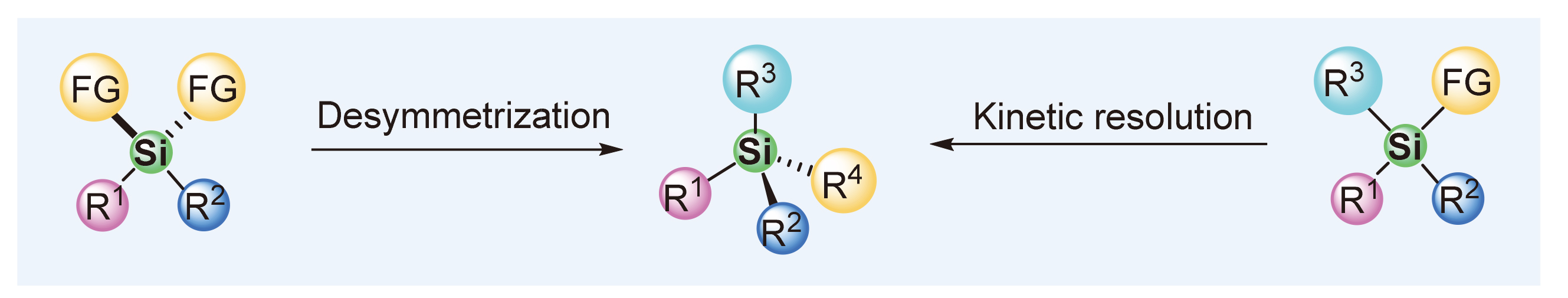
 About the Cover:The development of catalytic asymmetric Si—H/X—H dehydrogenative coupling reaction (Si-CADC) to construct Si-stereogenic centers is reviewed by Zhao, Ge and He on page 3352. By using dihydrosilanes as the substrates, the Si-CADC strategy has successfully realized the synthesis of various highly functionalized Si-stereogenic silanes with high efficiency and enantioselectivity. Inside Cover
About the Cover:The development of catalytic asymmetric Si—H/X—H dehydrogenative coupling reaction (Si-CADC) to construct Si-stereogenic centers is reviewed by Zhao, Ge and He on page 3352. By using dihydrosilanes as the substrates, the Si-CADC strategy has successfully realized the synthesis of various highly functionalized Si-stereogenic silanes with high efficiency and enantioselectivity. Inside Cover -
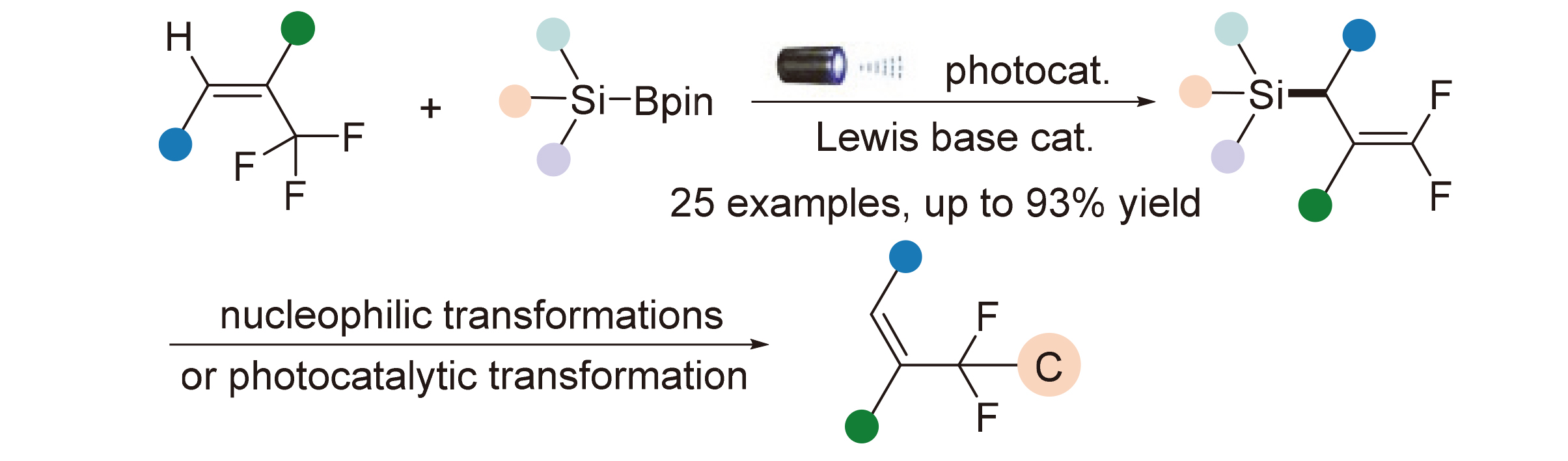
 About the Cover:The synthesis of β-silyl substituted vinylphosphine oxides by using the dialkynylphosphine oxides and silaboronate is reported by Xu and coworkers on page 3598. By means of constructing the carbon-silicon bonds, the synthesis of substituted vinylphosphine oxides was realized via copper-catalyzed mono-protosilylation of diynes, affording the products in moderate and high yields with high chemo- and regioselectivity.
About the Cover:The synthesis of β-silyl substituted vinylphosphine oxides by using the dialkynylphosphine oxides and silaboronate is reported by Xu and coworkers on page 3598. By means of constructing the carbon-silicon bonds, the synthesis of substituted vinylphosphine oxides was realized via copper-catalyzed mono-protosilylation of diynes, affording the products in moderate and high yields with high chemo- and regioselectivity. -
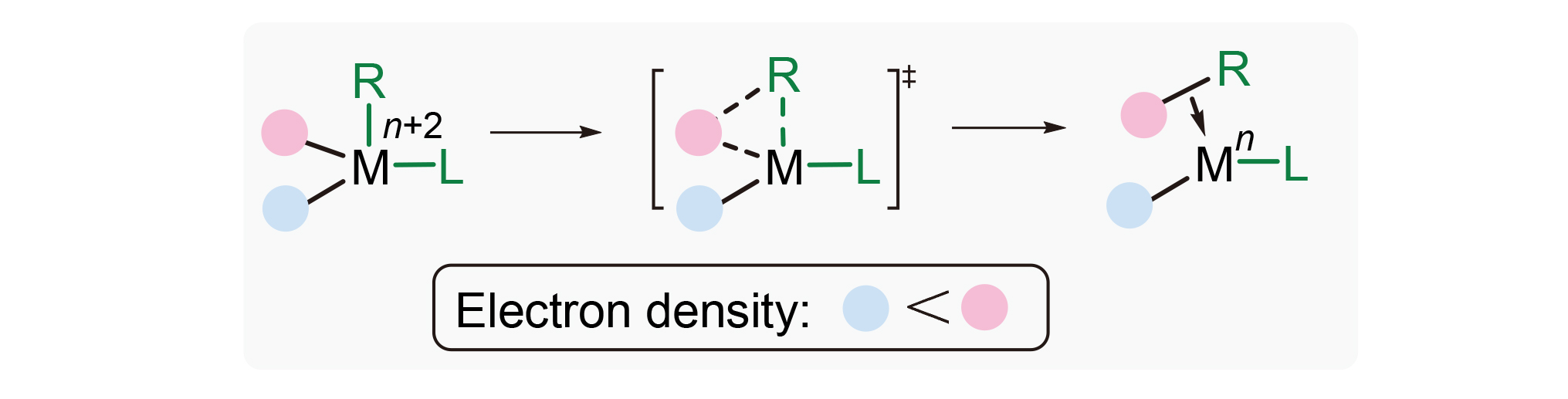
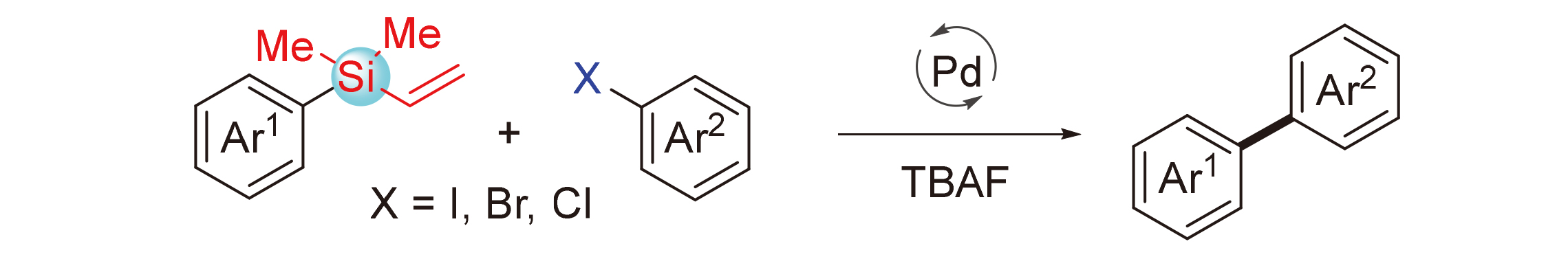
 About the Cover:Density functional theory (DFT) calculations revealing how electronic effect of substituent affects regioselectivity in C—Si reductive elimination step is reported by Peng, He, Liao, Bai and Lan on page 3608. Electron density of aromatic ring is the main factor to control regioselectivity. Aromatic rings with higher electron density have higher priority for C—Si reductive elimination of Pd(IV) intermediate, and this is in accordance with electron flow in this elementary reaction.
About the Cover:Density functional theory (DFT) calculations revealing how electronic effect of substituent affects regioselectivity in C—Si reductive elimination step is reported by Peng, He, Liao, Bai and Lan on page 3608. Electron density of aromatic ring is the main factor to control regioselectivity. Aromatic rings with higher electron density have higher priority for C—Si reductive elimination of Pd(IV) intermediate, and this is in accordance with electron flow in this elementary reaction.
-
-
Current Issue