化学学报 ›› 2021, Vol. 79 ›› Issue (4): 530-538.DOI: 10.6023/A20100468 上一篇 下一篇
研究论文
投稿日期:2020-10-13
发布日期:2021-02-22
通讯作者:
李蓓
基金资助:
Chang-An Liua,b, Shi-Bo Hongc, Bei Lia,b,*( )
)
Received:2020-10-13
Published:2021-02-22
Contact:
Bei Li
About author:Supported by:文章分享
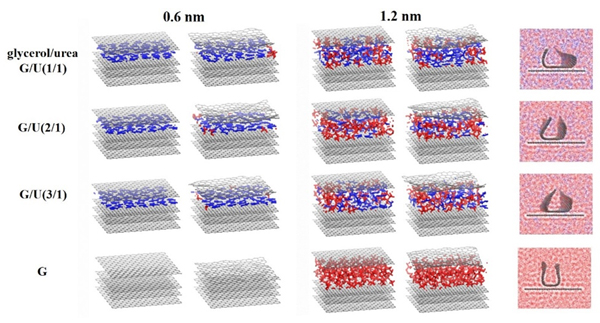
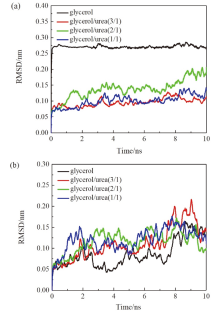
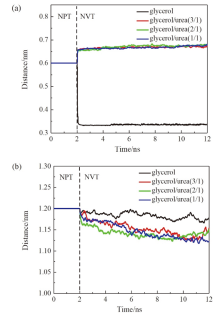
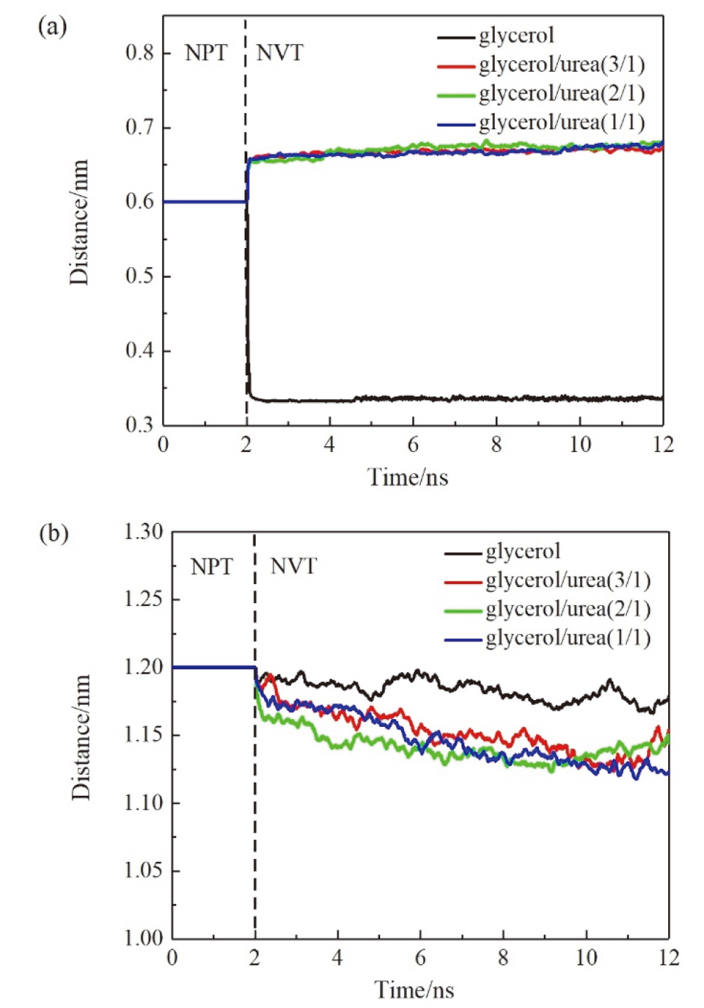
现有的实验方法很难实时观测到石墨烯在液相剥离溶剂中的结构演变, 尤其是石墨烯稳定的微观机理尚不明确. 本工作通过分子动力学方法, 模拟了多层石墨烯和U型石墨烯在不同的物质的量比下的甘油/尿素溶剂中的结构变化, 研究剥离液对石墨烯稳定性的影响. 结果表明, 多层石墨烯在不同溶剂体系中的稳定性差异不显著; 而U型石墨烯在各溶剂体系的稳定性有明显差异, 且稳定能力为: 纯甘油>甘油/尿素(2/1)>甘油/尿素(3/1)>甘油/尿素(1/1). 这说明石墨烯在剥离溶剂中的稳定性与石墨烯的剥离状态有关. 通过溶剂分布发现, 尿素能够进入石墨烯层间, 增加石墨烯层间距; 同时, 甘油能够与尿素形成氢键, 随尿素进入石墨烯层间, 进一步增大层间距, 从而形成稳定的单层或多层受限二元溶剂分子层. 受限溶剂分子层对剥离的石墨烯存在排斥作用, 从而为石墨烯在甘油/尿素二元剥离液中的长期稳定提供了保障.
刘长安, 洪士博, 李蓓. 石墨烯在甘油/尿素剥离液中的稳定行为的分子动力学模拟研究[J]. 化学学报, 2021, 79(4): 530-538.
Chang-An Liu, Shi-Bo Hong, Bei Li. Molecular Dynamics Simulation of the Stability Behavior of Graphene in Glycerol/Urea Solvents in Liquid-Phase Exfoliation[J]. Acta Chimica Sinica, 2021, 79(4): 530-538.


| 石墨烯尺寸/nm2 | 初始层间距/nm | 溶剂 | 甘油分子数 | 尿素分子数 | 模拟盒子初始尺寸/nm3 |
|---|---|---|---|---|---|
| 2.944×2.996 | 0.6 | 纯甘油 | 1432 | 0 | 6×6×6 |
| 甘油/尿素(3/1) | 1251 | 417 | |||
| 甘油/尿素(2/1) | 1142 | 571 | |||
| 甘油/尿素(1/1) | 935 | 935 | |||
| 纯甘油 | 1442 | 0 | |||
| 1.2 | 甘油/尿素(3/1) | 1257 | 419 | ||
| 甘油/尿素(2/1) | 1154 | 577 | |||
| 甘油/尿素(1/1) | 939 | 939 |
| 石墨烯尺寸/nm2 | 初始层间距/nm | 溶剂 | 甘油分子数 | 尿素分子数 | 模拟盒子初始尺寸/nm3 |
|---|---|---|---|---|---|
| 2.944×2.996 | 0.6 | 纯甘油 | 1432 | 0 | 6×6×6 |
| 甘油/尿素(3/1) | 1251 | 417 | |||
| 甘油/尿素(2/1) | 1142 | 571 | |||
| 甘油/尿素(1/1) | 935 | 935 | |||
| 纯甘油 | 1442 | 0 | |||
| 1.2 | 甘油/尿素(3/1) | 1257 | 419 | ||
| 甘油/尿素(2/1) | 1154 | 577 | |||
| 甘油/尿素(1/1) | 939 | 939 |
| 石墨烯尺寸/nm2 | 溶剂体系 | 甘油分子数 | 尿素分子数 | 模拟盒子初始尺寸/nm3 |
|---|---|---|---|---|
| 4.912×4.963 | 纯甘油 | 4227 | 0 | 9×9×9 |
| 甘油/尿素(3/1) | 3471 | 1157 | ||
| 甘油/尿素(2/1) | 3188 | 1594 | ||
| 甘油/尿素(1/1) | 2558 | 2558 |
| 石墨烯尺寸/nm2 | 溶剂体系 | 甘油分子数 | 尿素分子数 | 模拟盒子初始尺寸/nm3 |
|---|---|---|---|---|
| 4.912×4.963 | 纯甘油 | 4227 | 0 | 9×9×9 |
| 甘油/尿素(3/1) | 3471 | 1157 | ||
| 甘油/尿素(2/1) | 3188 | 1594 | ||
| 甘油/尿素(1/1) | 2558 | 2558 |



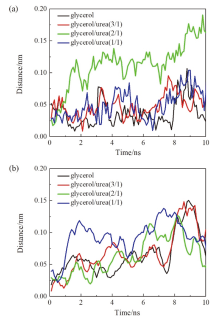




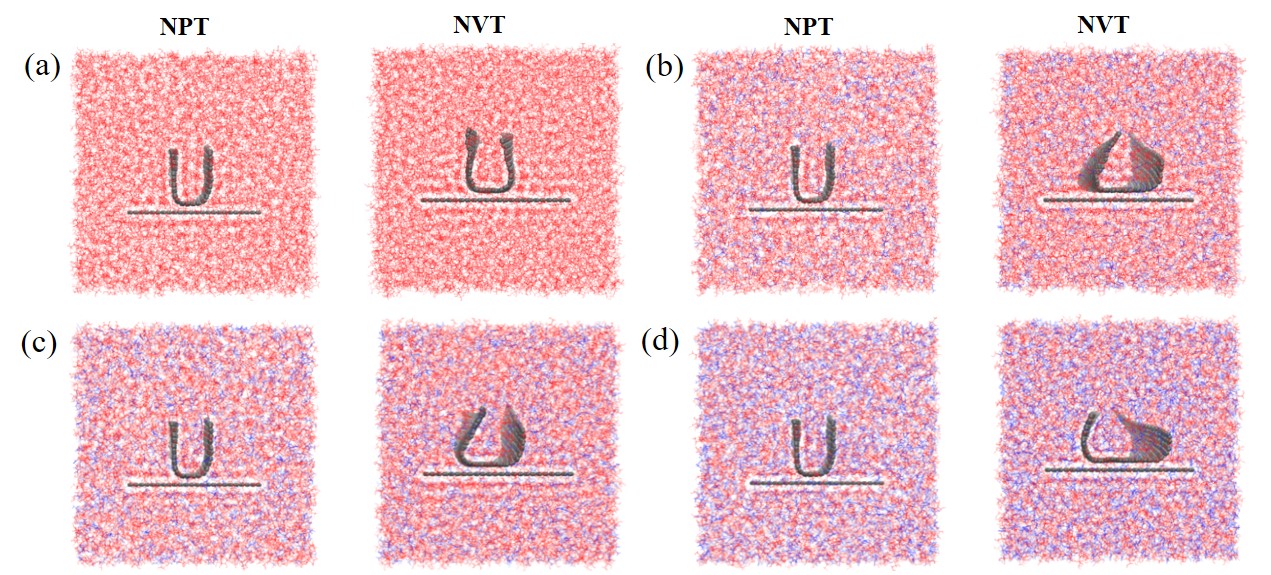
| [1] |
Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Science 2004, 306,666.
|
| [2] |
Hu, Y.J.; Jin, J.; Zhang, F.; Wu, P.; Cai, C.X. Acta Phys.-Chim. Sinica 2010, 26,2073 . . (in Chinese)
|
|
( 胡耀娟, 金娟, 张卉, 吴萍, 蔡称心, 物理化学学报, 2010, 26,2073.)
|
|
| [3] |
Fan, S.Q.; Tan, R.X.; Xie, X.M.; Zhang, M.Y.; Huang, Q.Z. New Carbon Mater. 2018, 33,522. (in Chinese)
|
|
( 樊姝婧, 谭瑞轩, 谢翔旻, 张明瑜, 黄启忠, 新型炭材料, 2018, 33,522.)
|
|
| [4] |
Le, M.Q. Int. J. Mech. Mater. Des. 2015, 11,15.
|
| [5] |
Li, X.Y.; Wang, J.; Wu, R. J. Chin. J. At.Mol. Phys. 2018, 35,139. (in Chinese)
|
|
( 李旭艳, 王静, 吴荣, 原子与分子物理学报, 2018, 35,139.)
|
|
| [6] |
Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Science 2008, 321,385.
|
| [7] |
Chen, J.H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Nat. Nanotechnol. 2008, 3,206.
|
| [8] |
Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Nano Lett. 2008, 8,902.
|
| [9] |
Liu, X. Ph.D. Dissertation, Donghua University, Shanghai, 2016. (in Chinese)
|
|
( 刘霞 , 博士论文, 东华大学, 上海, 2016.)
|
|
| [10] |
Walker, L.S.; Marotto, V.R.; Rafiee, M.A.; Koratkar, N.; Corral, E.L. ACS Nano 2011, 5,3182.
|
| [11] |
Kim, H.; Abdala, A.A.; Macosko, C.W. Macromolecules 2010, 43,6515.
|
| [12] |
Bai, H.; Li, C.; Shi, G. Adv. Mater. 2011, 23,1089.
|
| [13] |
Geng, Z.; Hähnlein, B.; Granzner, R.; Auge, M.; Ledeveb, A.A.; Davydov, V.Y.; Kitller, M.; Pezoldt, J.; Schwierz, F. Ann. Phys. 2017, 529,1700033.
|
| [14] |
Wang, H.; Kurata, K.; Fukunaga, T.; Ago, H.; Takamatsu, H.; Zhang, X.; Ikuta, T.; Takahashi, K.; Nishiyama, T.; Takata, Y. Sens. Actuators, A 2016, 247,24.
|
| [15] |
Ma, C. M.S. Thesis, Harbin Institute of Technology, Harbin, 2015. (in Chinese)
|
|
( 马聪 , 硕士论文, 哈尔滨工业大学, 哈尔滨, 2015.)
|
|
| [16] |
Tan, Y.B.; Lee, J.M. J. Mater. Chem. A 2013, 1,14814.
|
| [17] |
Liu, Y.; Tao, W.; Wu, D. Chin. J. Chem. 2020, 38,1123.
|
| [18] |
Hill, E.W.; Vijayaragahvan, A.; Novoselov, K. IEEE Sens. J. 2011, 11,3161.
|
| [19] |
Mackin, C.; Schroeder, V.; Zurutuza, A.; Su, C.; Kong, J.; Swager, T.M.; Palacios, T. ACS Appl. Mater. Interfaces 2018, 10,16169.
|
| [20] |
Miao, J.; Shi, Y.; Zhu, H.; Gao, M. Chin. J. Chem. 2020, 38,719.
|
| [21] |
Zhang, H.; Gruener, G.; Zhao, Y. J. Mater. Chem. B 2013, 1,2542.
|
| [22] |
Byun, J.J. Microbiol. Biotechnol. 2015, 25,145.
|
| [23] |
Lu, J.; Tan, S.; Zhu, Y.; Li, W.; Chen, T.; Wang, Y.; Liu, C. Acta Chim. Sinica 2019, 77,253. (in Chinese)
|
|
( 卢静荷, 谭淑珍, 朱雨清, 李伟, 陈天啸, 王瑶, 刘陈, 化学学报, 2019, 77,253.)
|
|
| [24] |
Song, G.; Wu, T.; Liu, F.; Zhang, B.; Liu, X. Acta Chim. Sinica 2020, 78,82. (in Chinese)
|
|
( 宋光捷, 武调弟, 刘福鑫, 张彬雁, 刘秀辉, 化学学报, 2020, 78,82.)
|
|
| [25] |
Meyer, J.C.; Geim, A.K.; Katsnelson, M.I.; Novoselov, K.S.; Booth, T.J.; Roth, S. Nature 2007, 446,60.
|
| [26] |
Sutter, P.W.; Flege, J.I. Nat. Mater. 2008, 7,406.
|
| [27] |
Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Rohrl, J.; Rotenberg, E.; Schmid, A.K.; Waldmann, D.; Weber, H.B.; Seyller, T. Nat. Mater. 2009, 8,203.
|
| [28] |
Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; Banerjee, S.K.; Colombo, L.; Ruoff, R.S. Science 2009, 324,1312.
|
| [29] |
Chae, S.J.; Güneş, F.; Kim, K.K.; Kim, E.S.; Han, G.E.; Kim, S.M.; Shin, H.; Yoon, S.; Choi, J.; Park, M.H.; Yang, C.W; Pribat, D.; Lee, Y.H. Adv. Mater. 2009, 21,2328.
|
| [30] |
Zhu, Y.; Stoller, M.D.; Cai, W.; Velamakanni, A.; Piner, R.D.; Chen., D.; Ruoff,, R.S . ACS Nano 2010, 4,1227.
|
| [31] |
Chen, W.; Yan, L. Nanoscale 2010, 2,559.
|
| [32] |
Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; GunKo, Y.K.; Boland, J.J.; Niraj, P.; Duesberg, G.; Krishnamurthy, S.; Goodhue, R.; Hutchison, J.; Scardaci, V.; Ferrari, A.C.; Coleman, J.N. Nat. Nanotechnol. 2008, 3,563.
|
| [33] |
Hernandez, Y.; Lotya, M.; Rickard, D.; Bergin, S.D.; Coleman, J.N. Langmuir 2010, 26,3208.
|
| [34] |
Lotya, M.; Hernandez, Y.; King, P.J.; Smith, R.J.; Nicolosi, V.; Karlsson, L.S.; Blighe, F.M.; De, S.; Wang, Z.; McGovern, I.T.; Duesberg, G.S.; Coleman, J.N. J. Am. Chem. Soc. 2009, 131,3611.
|
| [35] |
Coleman, J.N. Adv. Funct. Mater. 2009, 19,3680.
|
| [36] |
Coleman, J.N. Acc. Chem. Res. 2013, 46,14.
|
| [37] |
Shih, C.J.; Lin, S.; Strano, M.S.; Blankschtein, D. J. Am. Chem. Soc. 2010, 132,14638.
|
| [38] |
Fu, C.; Yang, X. Carbon 2013, 55,350.
|
| [39] |
Yang, J.; Yang, X.; Li, Y. Curr. Opin. Colloid Interface Sci. 2015, 20,339.
|
| [40] |
Xu, X.; Cai, L.; Zheng, X.; Xu, Q. Phys. Chem. Chem. Phys. 2017, 19,16062.
|
| [41] |
Chiu, P.L.; Mastrogiovanni, D.D. T.; Wei, D.; Louis, C.; Jeong, M.; Yu, G.; Saad, P.; Flach, C.R.; Mendelsohn, R.; Garfunkel, E.; He, H. J. Am. Chem. Soc. 2012, 134,5850.
|
| [42] |
Shi, M.Y.; Zhang, X.F.; Wang, X.Y.; Wang, W.Z.; Jiang, X.Q. J. Nanjing Norm. Univ. (Engineering and Technology) 2014, 14,1. (in Chinese)
|
|
( 石梦燕, 张晓凤, 王孝英, 王文珠, 蒋晓青, 南京师范大学学报: 工程技术版, 2014, 14,1.)
|
|
| [43] |
Chen, J.; Shi, W.; Gao, Z.; Wang, T.; Wang, S.; Dong, L.; Yang, Q.; Xiong, C. Nano Res. 2018, 11,820.
|
| [44] |
Kim, H.S.; Oweida, T.J.; Yingling, Y.G. J. Mater. Sci. 2018, 53,5766.
|
| [45] |
Kim, H.S.; Huang, S.M.; Yingling, Y.G. MRS Adv. 2016, 1,1883.
|
| [46] |
Kim, H.S.; Farmer, B.L.; Yingling, Y.G. Adv. Mater. Interfaces 2017, 4,1601168.
|
| [47] |
Sun, J.; Li, Y.; Lin, J. J. Mol. Graphics Modell. 2017, 74,16.
|
| [48] |
da Silva, A.W. S.; Vranken, W.F. BMC Res. Notes 2012, 5,367.
|
| [49] |
Mandell, M.J.; McTague, J.P.; Rahman, A. J. Chem. Phys. 1976, 64,3699.
|
| [50] |
Hess, B.; Bekker, H.; Berendsen, H.J. C.; Fraaije, J. G. E.M.. J. Comput. Chem. 1997, 18,1463.
|
| [51] |
Hess, B. J. Chem. Theory Comput. 2008, 4,116.
|
| [52] |
Darden, T.; York, D.; Pedersen, L. J. Chem. Phys. 1998, 98,10089.
|
| [53] |
Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. J. Chem. Phys. 1995, 103,8577.
|
| [54] |
Yau, A.W.; Pritchard, H.O. Can. J. Chem. 1977, 55,992.
|
| [55] |
Berendsen, H.J. C.; Postma, J.P. M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. J. Chem. Phys. 1984, 81,3684.
|
| [56] |
Bussi, G.; Donadio, D.; Parrinello, M. J. Chem. Phys. 2007, 126,14101.
|
| [57] |
Parrinello, M.; Rahman, A. J. Appl. Phys. 1981, 52,7182.
|
| [58] |
Hoover, W.G. Phys. Rev. A 1985, 31,1695.
|
| [59] |
Jussila, H.; Yang, H.; Granqvist, N.; Sun, Z. Optica 2016, 3,151.
|
| [60] |
Wolfe, M.; Jonas, J. J. Chem. Phys. 1979, 71,3252.
|
| [61] |
Lide, D.R. CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data, CRC Press, New York, 1995.
|
| [62] |
Carugo, O.; Pongor, S. Protein Sci. 2001, 10,1470.
|
| [63] |
Moghaddam, M.B.; Goharshadi, E.K.; Entezari, M.H.; Nancarrow, P. Chem. Eng. J. 2013, 231,365.
|
| [64] |
Li, B.; Hong, S.; Zhang, X.; Xiong, C.; Zhao, G.; Yang, Q.; Liu, H. Adv. Theory Simul. 2019, 2,1900155.
|
| [1] | 王海朋, 蔡文生, 邵学广. 抗冻剂抗冻机制的近红外光谱与分子模拟研究★[J]. 化学学报, 2023, 81(9): 1167-1174. |
| [2] | 苑志祥, 张浩, 胡思伽, 张波涛, 张建军, 崔光磊. 离子聚合原位固态化构建高安全锂电池固态聚合物电解质的研究进展★[J]. 化学学报, 2023, 81(8): 1064-1080. |
| [3] | 刘祯钰, 甘利华. 乙炔热解为富勒烯的分子动力学模拟研究[J]. 化学学报, 2023, 81(5): 502-510. |
| [4] | 宁聪聪, 杨倩, 毛阿敏, 唐梓嘉, 金燕, 胡宝山. 石墨烯纳米带的可控制备研究进展[J]. 化学学报, 2023, 81(4): 406-419. |
| [5] | 高丰琴, 刘洋, 张引莉, 蒋育澄. 羧基功能化Fe3O4固定化酶反应器的构筑及性能研究[J]. 化学学报, 2023, 81(4): 338-344. |
| [6] | 姜兰, 范义秋, 张晓昕, 裴燕, 闫世润, 乔明华, 范康年, 宗保宁. W量对Pt/GaWZrOx催化剂结构及甘油选择氢解性能的影响[J]. 化学学报, 2023, 81(3): 231-238. |
| [7] | 查汉, 房进, 闫翎鹏, 杨永珍, 马昌期. 有机太阳能电池热失效机制及三元共混提升其热稳定性研究进展[J]. 化学学报, 2023, 81(2): 131-145. |
| [8] | 韩逸之, 蓝建慧, 刘学, 石伟群. 基于机器学习势函数的熔盐体系分子动力学研究进展[J]. 化学学报, 2023, 81(11): 1663-1672. |
| [9] | 赵珂, 程夏宇, 马雪雪, 耿明慧. 含哌嗪基团锌离子探针的双光子吸收增强机理[J]. 化学学报, 2023, 81(10): 1371-1378. |
| [10] | 刘稳, 王昱捷, 杨慧琴, 李成杰, 吴娜, 颜洋. 离子液体非共价诱导制备碳纳米管/石墨烯集流体用于钠金属负极[J]. 化学学报, 2023, 81(10): 1379-1386. |
| [11] | 闫绍兵, 焦龙, 何传新, 江海龙. ZIF-67/石墨烯复合物衍生的氮掺杂碳限域Co纳米颗粒用于高效电催化氧还原[J]. 化学学报, 2022, 80(8): 1084-1090. |
| [12] | 刘彦甫, 李世麟, 荆亚楠, 肖林格, 周惠琼. 有机太阳能电池性能衰减机理研究进展[J]. 化学学报, 2022, 80(7): 993-1009. |
| [13] | 曾杨, 姜兰, 张晓昕, 谢颂海, 裴燕, 乔明华, 李振华, 徐华龙, 范康年, 宗保宁. W掺杂多级孔SiO2纳米球负载Pt用于催化甘油氢解制1,3-丙二醇[J]. 化学学报, 2022, 80(7): 903-912. |
| [14] | 许宁, 乔庆龙, 刘晓刚, 徐兆超. 基于抑制扭转的分子内电荷转移(TICT)提升有机小分子荧光染料亮度及光稳定性※[J]. 化学学报, 2022, 80(4): 553-562. |
| [15] | 戴敏, 雷钢铁, 张钊, 李智, 曹湖军, 陈萍. 五氧化二钒促进MgH2/Mg室温吸氢※[J]. 化学学报, 2022, 80(3): 303-309. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||