化学学报 ›› 2022, Vol. 80 ›› Issue (6): 708-713.DOI: 10.6023/A22010054 上一篇 下一篇
研究论文
栾雪菲a,b, 王聪芝b, 夏良树a,*( ), 石伟群b,*(
), 石伟群b,*( )
)
投稿日期:2022-01-28
发布日期:2022-07-07
通讯作者:
夏良树, 石伟群
基金资助:
Xuefei Luana,b, Congzhi Wangb, Liangshu Xiaa( ), Weiqun Shib(
), Weiqun Shib( )
)
Received:2022-01-28
Published:2022-07-07
Contact:
Liangshu Xia, Weiqun Shi
Supported by:文章分享

深入了解各种功能基团与铀酰离子的络合行为有助于设计和开发高效海水提铀吸附剂. 本工作通过密度泛函理论(DFT)方法系统地研究了两种典型铀酰络合配体吡啶-2,6-二羧酸(H2DPA)和戊二酰偕亚胺二肟(H2A)与铀酰离子及碳酸根离子形成的配合物的结构、成键性质以及热力学稳定性. 研究结果表明, 所有配合物中, 配体与铀酰离子之间具有不同强度的共价相互作用. 由于H2A配位时发生了质子重排, 而且配体的解离能较高, 使其更难与[UO2(CO3)3]4-发生取代反应, 因此H2DPA配体是海水提铀中一种潜在的有效配体. 本工作的相关研究结果为海水提铀中高效吸附基团的设计和开发提供了理论线索.
栾雪菲, 王聪芝, 夏良树, 石伟群. 铀酰与羧酸和肟基类配体相互作用的理论研究[J]. 化学学报, 2022, 80(6): 708-713.
Xuefei Luan, Congzhi Wang, Liangshu Xia, Weiqun Shi. Theoretical Studies on the Interaction of Uranyl with Carboxylic Acids and Oxime Ligands[J]. Acta Chimica Sinica, 2022, 80(6): 708-713.
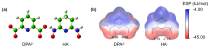
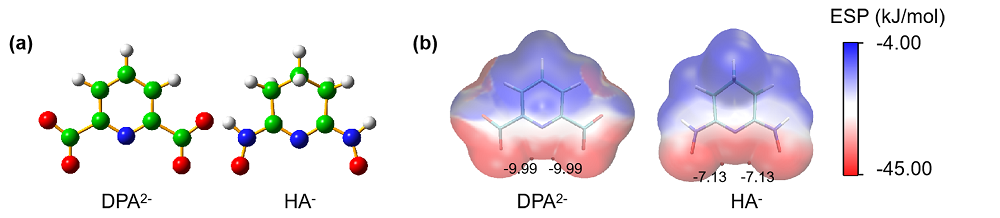
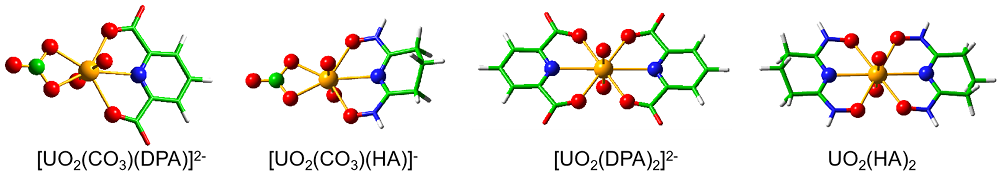
| 铀酰配合物 | U=Oax | U—N(L) | U—O(L) | U—O(CO32-) | vs | vas |
|---|---|---|---|---|---|---|
| [UO2(CO3)3]4- | 0.182 | 0.253 | 765.58 | 835.01 | ||
| [UO2(CO3)(DPA)]2- | 0.180 | 0.265 | 0.247 | 0.231 | 826.61 | 893.72 |
| [UO2(CO3)(HA)]- | 0.180 | 0.266 | 0.254 | 0.228 | 825.19 | 895.12 |
| [UO2(DPA)2]2- | 0.178 | 0.276 | 0.248 | 848.10 | 929.87 | |
| UO2(HA)2 | 0.178 | 0.266 | 0.250 | 851.62 | 932.91 |
| 铀酰配合物 | U=Oax | U—N(L) | U—O(L) | U—O(CO32-) | vs | vas |
|---|---|---|---|---|---|---|
| [UO2(CO3)3]4- | 0.182 | 0.253 | 765.58 | 835.01 | ||
| [UO2(CO3)(DPA)]2- | 0.180 | 0.265 | 0.247 | 0.231 | 826.61 | 893.72 |
| [UO2(CO3)(HA)]- | 0.180 | 0.266 | 0.254 | 0.228 | 825.19 | 895.12 |
| [UO2(DPA)2]2- | 0.178 | 0.276 | 0.248 | 848.10 | 929.87 | |
| UO2(HA)2 | 0.178 | 0.266 | 0.250 | 851.62 | 932.91 |
| 铀酰配合物 | U—N(L) | U—O(L) | U—O(CO32-) | Q(U) | ΔQ(L) | ΔQ(CO32-) |
|---|---|---|---|---|---|---|
| [UO2(CO3)(DPA)]2- | 0.301 | 0.449 | 0.700 | 1.544 | 0.716 | 0.897 |
| [UO2(CO3)(HA)]- | 0.281 | 0.401 | 0.749 | 1.554 | 0.647 | 0.958 |
| [UO2(DPA)2]2- | 0.301 | 0.480 | 1.499 | 1.574 | ||
| UO2(HA)2 | 0.335 | 0.475 | 1.471 | 1.593 |
| 铀酰配合物 | U—N(L) | U—O(L) | U—O(CO32-) | Q(U) | ΔQ(L) | ΔQ(CO32-) |
|---|---|---|---|---|---|---|
| [UO2(CO3)(DPA)]2- | 0.301 | 0.449 | 0.700 | 1.544 | 0.716 | 0.897 |
| [UO2(CO3)(HA)]- | 0.281 | 0.401 | 0.749 | 1.554 | 0.647 | 0.958 |
| [UO2(DPA)2]2- | 0.301 | 0.480 | 1.499 | 1.574 | ||
| UO2(HA)2 | 0.335 | 0.475 | 1.471 | 1.593 |
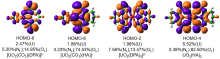
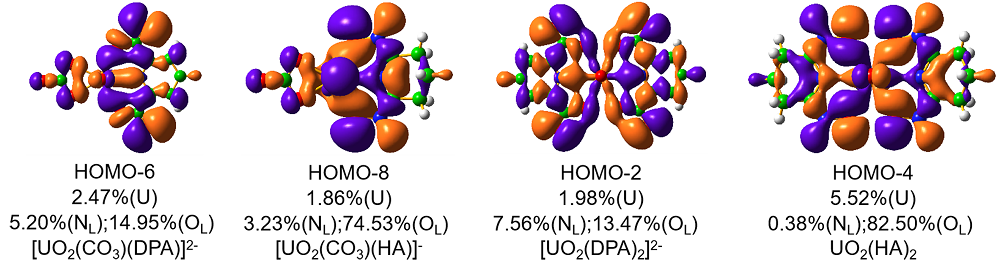

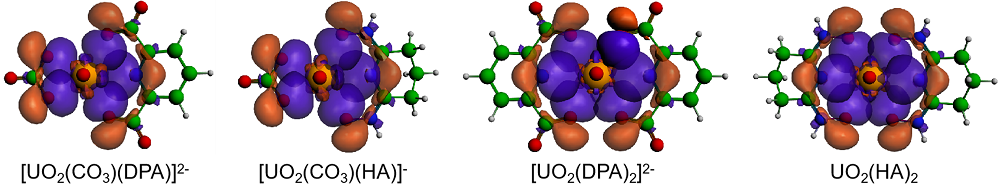
| 铀酰配合物 | ΔEPauli | ΔEelstat | ΔEorb | ΔEint | %ΔEelstat | %ΔEorb |
|---|---|---|---|---|---|---|
| [UO2(CO3)(DPA)]2- | 1017.7 | –3837.3 | –1385.6 | –4205.1 | 73.47 | 26.53 |
| [UO2(CO3)(HA)]- | 1035.6 | –3311.6 | –1403.1 | –3679.0 | 70.24 | 29.76 |
| [UO2(DPA)2]2- | 664.2 | –3518.4 | –1179.6 | –4033.8 | 74.89 | 25.11 |
| UO2(HA)2 | 705.9 | –2563.6 | –1187.1 | –3044.8 | 68.35 | 31.65 |
| 铀酰配合物 | ΔEPauli | ΔEelstat | ΔEorb | ΔEint | %ΔEelstat | %ΔEorb |
|---|---|---|---|---|---|---|
| [UO2(CO3)(DPA)]2- | 1017.7 | –3837.3 | –1385.6 | –4205.1 | 73.47 | 26.53 |
| [UO2(CO3)(HA)]- | 1035.6 | –3311.6 | –1403.1 | –3679.0 | 70.24 | 29.76 |
| [UO2(DPA)2]2- | 664.2 | –3518.4 | –1179.6 | –4033.8 | 74.89 | 25.11 |
| UO2(HA)2 | 705.9 | –2563.6 | –1187.1 | –3044.8 | 68.35 | 31.65 |
| 络合反应 | ΔGBE |
|---|---|
| [UO2(H2O)5]2++CO32-+DPA2-→[UO2(CO3)(DPA)]2-+5H2O | –452.1 |
| [UO2(H2O)5]2++2DPA2-→[UO2(DPA)2]2-+5H2O | –363.3 |
| [UO2(H2O)5]2++CO32-+HA-→[UO2(CO3)(HA)] -+5H2O | –472.6 |
| [UO2(H2O)5]2++2HA-→UO2(HA)2+5H2O | –446.6 |
| 络合反应 | ΔGBE |
|---|---|
| [UO2(H2O)5]2++CO32-+DPA2-→[UO2(CO3)(DPA)]2-+5H2O | –452.1 |
| [UO2(H2O)5]2++2DPA2-→[UO2(DPA)2]2-+5H2O | –363.3 |
| [UO2(H2O)5]2++CO32-+HA-→[UO2(CO3)(HA)] -+5H2O | –472.6 |
| [UO2(H2O)5]2++2HA-→UO2(HA)2+5H2O | –446.6 |
| 取代反应 | ΔG |
|---|---|
| [UO2(CO3)3]4-+H2DPA→[UO2(CO3)(DPA)]2-+2HCO3- | –103.0 |
| [UO2(CO3)(DPA)]2-+H2DPA→[UO2(DPA)2]2-+HCO3-+H+ | 41.4 |
| [UO2(CO3)3]4-+2H2DPA→[UO2(DPA)2]2-+3HCO3-+H+ | –61.1 |
| [UO2(CO3)3]4-+H2A→[UO2(CO3)(HA)] -+HCO3-+CO32- | 137.3 |
| [UO2(CO3)(HA)] -+H2A→UO2(HA)2+HCO3- | 8.4 |
| [UO2(CO3)3]4-+2H2A→UO2(HA)2+2HCO3-+CO32- | 145.7 |
| 取代反应 | ΔG |
|---|---|
| [UO2(CO3)3]4-+H2DPA→[UO2(CO3)(DPA)]2-+2HCO3- | –103.0 |
| [UO2(CO3)(DPA)]2-+H2DPA→[UO2(DPA)2]2-+HCO3-+H+ | 41.4 |
| [UO2(CO3)3]4-+2H2DPA→[UO2(DPA)2]2-+3HCO3-+H+ | –61.1 |
| [UO2(CO3)3]4-+H2A→[UO2(CO3)(HA)] -+HCO3-+CO32- | 137.3 |
| [UO2(CO3)(HA)] -+H2A→UO2(HA)2+HCO3- | 8.4 |
| [UO2(CO3)3]4-+2H2A→UO2(HA)2+2HCO3-+CO32- | 145.7 |
| [1] |
Li, H.; Wen, J.; Wang, X.-L. Chinese Sci. Bull. 2018, 63, 481. (in Chinese)
doi: 10.1360/N972017-01122 |
|
(李昊, 文君, 汪小琳, 科学通报, 2018, 63, 481.)
|
|
| [2] |
Hu, B.; Wang, H.; Liu, R.; Qiu, M. Chemosphere 2021, 274, 129743.
doi: 10.1016/j.chemosphere.2021.129743 |
| [3] |
Endrizzi, F.; Leggett, C. J.; Rao, L. Ind. Eng. Chem. Res. 2016, 55, 4249.
doi: 10.1021/acs.iecr.5b03679 |
| [4] |
Parker, B. F.; Hohloch, S.; Pankhurst, J. R.; Zhang, Z.; Love, J. B.; Arnold, J.; Rao, L. Dalton Trans. 2018, 47, 5695.
doi: 10.1039/c7dt04069e pmid: 29632905 |
| [5] |
Yuan, Y. H.; Niu, B. Y.; Yu, Q. H.; Guo, X.; Guo, Z. H.; Wen, J.; Liu, T.; Zhang, H. Q.; Wang, N. Angew. Chem.-Int. Ed. 2020, 59, 1220.
doi: 10.1002/anie.201913644 |
| [6] |
Tian, G.; Geng, J. X.; Jin, Y. D.; Wang, C. L.; Li, S. Q.; Chen, Z.; Wang, H.; Zhao, Y. S.; Li, S. J. J. Hazard. Mater. 2011, 190, 442.
doi: 10.1016/j.jhazmat.2011.03.066 pmid: 21497013 |
| [7] |
Zhu, J. H.; Liu, Q.; Li, Z. S.; Liu, J. Y.; Zhang, H. S.; Li, R. M.; Wang, J. J. Hazard. Mater. 2018, 353, 9.
doi: 10.1016/j.jhazmat.2018.03.042 |
| [8] |
Wang, C. Z.; Lan, J. H.; Wu, Q. Y.; Luo, Q.; Zhao, Y. L.; Wang, X. K.; Chai, Z. F.; Shi, W. Q. Inorg. Chem. 2014, 53, 9466.
doi: 10.1021/ic500202g |
| [9] |
Li, Z.-N.; Sha, H.-Y.; Yang, N.; Yuan, Y.; Zhu, G.-S. Acta Chim. Sinica 2019, 77, 469. (in Chinese)
doi: 10.6023/A19010028 |
|
(李樟楠, 沙浩岩, 杨南, 元野, 朱广山, 化学学报, 2019, 77, 469.)
doi: 10.6023/A19010028 |
|
| [10] |
Abney, C. W.; Mayes, R. T.; Saito, T.; Dai, S. Chem. Rev. 2017, 117, 13935.
doi: 10.1021/acs.chemrev.7b00355 |
| [11] |
Parker, B. F.; Zhang, Z.; Rao, L.; Arnold, J. Dalton Trans. 2018, 47, 639.
doi: 10.1039/c7dt04058j pmid: 29261203 |
| [12] |
Liu, Z.-Y.; Xie, Y.; Wang, Y.-F.; Hu, T.-Y.; Ye, G.; Chen, J. J. Tsinghua Univ. (Sci. & Technol.) 2021, 61, 279. (in Chinese)
|
|
(刘泽宇, 谢忆, 王一凡, 胡铜洋, 叶钢, 陈靖, 清华大学学报(自然科学版), 2021, 61, 279.)
|
|
| [13] |
Tang, N.; Liang, J.; Niu, C. G.; Wang, H.; Luo, Y.; Xing, W. L.; Ye, S. J.; Liang, C.; Guo, H.; Guo, J. Y.; Zhang, Y. F.; Zeng, G. M. J. Mater. Chem. A 2020, 8, 7588.
doi: 10.1039/C9TA14082D |
| [14] |
Sun, Q.; Aguila, B.; Earl, L. D.; Abney, C. W.; Wojtas, L.; Thallapally, P. K.; Ma, S. Adv. Mater. 2018, 30, e1705479.
|
| [15] |
Zhang, L.; Pu, N.; Yu, B.; Ye, G.; Chen, J.; Xu, S.; Ma, S. ACS Appl. Mater. Interfaces 2020, 12, 3688.
doi: 10.1021/acsami.9b20944 |
| [16] |
Xu, X.; Xu, L.; Ao, J.; Liang, Y.; Li, C.; Wang, Y.; Huang, C.; Ye, F.; Li, Q.; Guo, X.; Li, J.; Wang, H.; Ma, S.; Ma, H. J. Mater. Chem. A 2020, 8, 22032.
doi: 10.1039/D0TA07180C |
| [17] |
Tian, G. X.; Teat, S. J.; Zhang, Z. Y.; Rao, L. F. Dalton Trans. 2012, 41, 11579.
doi: 10.1039/c2dt30978e |
| [18] |
Xu, C.; Tian, G.; Teat, S. J.; Rao, L. Inorg. Chem. 2013, 52, 2750.
doi: 10.1021/ic4000389 |
| [19] |
Zhou, D.; Huang, C.; Wang, K.; Xu, G. Polyhedron 1994, 13, 987.
doi: 10.1016/S0277-5387(00)83020-X |
| [20] |
Guo, X.; Huang, L.; Li, C.; Hu, J.; Wu, G.; Huai, P. Phys. Chem. Chem. Phys. 2015, 17, 14662.
doi: 10.1039/C5CP00931F |
| [21] |
Murray, J. S.; Politzer, P. Wiley Interdiscip. Rev.-Comput. Mol. Sci. 2011, 1, 153.
doi: 10.1002/wcms.19 |
| [22] |
Pyykkö, P.; Li, J.; Runeberg, N. J. Phys. Chem. 1994, 98, 4809.
doi: 10.1021/j100069a007 |
| [23] |
Perdew, J. P.; Burke, K.; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865.
doi: 10.1103/PhysRevLett.77.3865 pmid: 10062328 |
| [24] |
Moellmann, J.; Grimme, S. J. Phys. Chem. C 2014, 118, 7615.
doi: 10.1021/jp501237c |
| [25] |
Wiberg, K. B. J. Am. Chem. Soc. 1968, 90, 59.
doi: 10.1021/ja01003a012 |
| [26] |
Reed, A. E.; Weinstock, R. B.; Weinhold, F. J. Chem. Phys. 1985, 83, 735.
doi: 10.1063/1.449486 |
| [27] |
Ziegler, T.; Rauk, A. Theor. Chim. Acta 1977, 46, 1.
doi: 10.1007/BF02401406 |
| [28] |
Sun, X. Q.; Xu, C.; Tian, G. X.; Rao, L. F. Dalton Trans. 2013, 42, 14621.
doi: 10.1039/c3dt51767e |
| [29] |
Tian, G.; Teat, S. J.; Rao, L. Dalton Trans. 2013, 42, 5690.
doi: 10.1039/c3dt32940b |
| [30] |
Bernhard, G.; Geipel, G.; Reich, T.; Brendler, V.; Amayri, S.; Nitsche, H. Radiochim. Acta 2001, 89, 511.
doi: 10.1524/ract.2001.89.8.511 |
| [31] |
Kelly, S. D.; Kemner, K. M.; Brooks, S. C. Geochim. Cosmochim. Acta 2007, 71, 821.
doi: 10.1016/j.gca.2006.10.013 |
| [32] |
Becke, A. D. J. Chem. Phys. 1993, 98, 5648.
doi: 10.1063/1.464913 |
| [33] |
Lee, C. T.; Yang, W. T.; Parr, R. G. Phys. Rev. B 1988, 37, 785.
pmid: 9944570 |
| [34] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J. A., Jr; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 1, Revision B. 01,Gaussian Inc., Wallingford, CT, 2016.
|
| [35] |
Andrae, D.; Haussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Theor. Chim. Acta 1990, 77, 123.
doi: 10.1007/BF01114537 |
| [36] |
Dolg, M.; Wedig, U.; Stoll, H.; Preuss, H. J. Chem. Phys. 1987, 86, 866.
doi: 10.1063/1.452288 |
| [37] |
Yang, C.; Pei, S.; Chen, B.; Ye, L.; Yu, H.; Hu, S. Dalton Trans. 2016, 45, 3120.
doi: 10.1039/C5DT04645A |
| [38] |
Guo, X.; Xiong, X.-G.; Li, C.; Gong, H.; Huai, P.; Hu, J.; Jin, C.; Huang, L.; Wu, G. Inorg. Chim. Acta 2016, 441, 117.
doi: 10.1016/j.ica.2015.11.013 |
| [39] |
Luan, X.-F.; Wang, C.-Z.; Wu, Q.-Y.; Lan, J.-H.; Chai, Z.-F.; Xia, L.-S.; Shi, W.-Q. J. Phys. Chem. A 2022, 126, 406.
doi: 10.1021/acs.jpca.1c08072 |
| [40] |
Baldridge, K.; Klamt, A. J. Chem. Phys. 1997, 106, 6622.
doi: 10.1063/1.473662 |
| [41] |
Andzelm, J.; Kolmel, C.; Klamt, A. J. Chem. Phys. 1995, 103, 9312.
doi: 10.1063/1.469990 |
| [42] |
Barone, V.; Cossi, M. J. Phys. Chem. A 1998, 102, 1995.
doi: 10.1021/jp9716997 |
| [43] |
Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. J. Comput. Chem. 2003, 24, 669.
doi: 10.1002/jcc.10189 |
| [44] |
Schreckenbach, G.; Shamov, G. A. Acc. Chem. Res. 2010, 43, 19.
doi: 10.1021/ar800271r |
| [45] |
Shamov, G. A.; Schreckenbach, G. J. Phys. Chem. A 2005, 109, 10961.
doi: 10.1021/jp053522f |
| [46] |
Camaioni, D. M.; Schwerdtfeger, C. A. J. Phys. Chem. A 2005, 109, 10795.
pmid: 16863129 |
| [47] |
te Velde, G.; Bickelhaupt, F. M.; Baerends, E. J.; Guerra, C. F.; Van Gisbergen, S. J. A.; Snijders, J. G.; Ziegler, T. J. Comput. Chem. 2001, 22, 931.
doi: 10.1002/jcc.1056 |
| [48] |
Guerra, C. F.; Snijders, J. G.; te Velde, G.; Baerends, E. J. Theor. Chem. Acc. 1998, 99, 391.
|
| [49] |
Vanlenthe, E.; Baerends, E. J.; Snijders, J. G. J. Chem. Phys. 1993, 99, 4597.
doi: 10.1063/1.466059 |
| [50] |
Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. 1988, 88, 899.
doi: 10.1021/cr00088a005 |
| [51] |
Lu, T.; Chen, F. W. J. Comput. Chem. 2012, 33, 580.
doi: 10.1002/jcc.22885 |
| [1] | 黄广龙, 薛小松. “陈试剂”作为三氟甲基源机理的理论研究[J]. 化学学报, 2024, 82(2): 132-137. |
| [2] | 梁雪峰, 荆剑, 冯昕, 赵勇泽, 唐新员, 何燕, 张立胜, 李慧芳. 共价有机框架COF66/COF366的电子结构: 从单体到二维平面聚合物[J]. 化学学报, 2023, 81(7): 717-724. |
| [3] | 杨磊, 葛娇阳, 王访丽, 吴汪洋, 郑宗祥, 曹洪涛, 王洲, 冉雪芹, 解令海. 一种基于芴的大环结构的有效降低内重组能的理论研究[J]. 化学学报, 2023, 81(6): 613-619. |
| [4] | 张少秦, 李美清, 周中军, 曲泽星. 多共振热激活延迟荧光过程的理论研究[J]. 化学学报, 2023, 81(2): 124-130. |
| [5] | 王娟, 肖华敏, 谢丁, 郭元茹, 潘清江. 铜掺杂与氮化碳复合氧化锌材料结构和二氧化氮气体传感性质的密度泛函理论计算[J]. 化学学报, 2023, 81(11): 1493-1499. |
| [6] | 刘金晶, 杨娜, 李莉, 魏子栋. 铂活性位空间结构调控氧还原机理的理论研究★[J]. 化学学报, 2023, 81(11): 1478-1485. |
| [7] | 王珞聪, 李哲伟, 岳彩巍, 张培焕, 雷鸣, 蒲敏. 电场下偶氮苯衍生物分子顺反异构化反应机理的理论研究[J]. 化学学报, 2022, 80(6): 781-787. |
| [8] | 熊昆, 陈伽瑶, 杨娜, 蒋尚坤, 李莉, 魏子栋. 理论探究水溶液条件对TMNxCy催化氮还原性能的增强机制[J]. 化学学报, 2021, 79(9): 1138-1145. |
| [9] | 王英辉, 魏思敏, 段金伟, 王康. 理论研究“受阻路易斯酸碱对”催化的烯醇硅醚氢化反应机理[J]. 化学学报, 2021, 79(9): 1164-1172. |
| [10] | 满清敏, 付尊蕴, 刘甜甜, 郑明月, 蒋华良. Cu催化偶联反应合成烷基芳基醚的DFT机理研究[J]. 化学学报, 2021, 79(7): 948-952. |
| [11] | 王岩, 田英齐, 金钟, 索兵兵. 基于GPU的Hartree-Fock与密度泛函算法及程序[J]. 化学学报, 2021, 79(5): 653-657. |
| [12] | 张丹琪, 邵英博, 郑汉良, 周碧莹, 薛小松. 双齿氮配体螯合五价碘试剂介导的苯酚氧化去芳构化机理的理论研究[J]. 化学学报, 2021, 79(11): 1394-1400. |
| [13] | 鲁效庆, 曹守福, 魏晓飞, 李邵仁, 魏淑贤. S掺杂Fe-NC单原子催化剂氧还原机理研究[J]. 化学学报, 2020, 78(9): 1001-1006. |
| [14] | 于沫涵, 程媛媛, 刘亚军. 萤火虫生物发光中加氧反应机理的理论研究[J]. 化学学报, 2020, 78(9): 989-993. |
| [15] | 李哲伟, 王骞阅, 蒲敏, 杨作银, 雷鸣. 含氮杂环协助的醛胺缩合反应机理的研究[J]. 化学学报, 2020, 78(5): 437-443. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||