有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1670-1682.DOI: 10.6023/cjoc202008057 上一篇 下一篇
研究论文
漆亚云a, 刘佳敏a, 李成朋a, 胡伟男a, 唐思雨a, 邵利辉a, 王贞超a,b,*( ), 欧阳贵平a,b,c,*(
), 欧阳贵平a,b,c,*( )
)
收稿日期:2020-08-31
修回日期:2020-11-06
发布日期:2020-12-10
通讯作者:
王贞超, 欧阳贵平
作者简介:基金资助:
Yayun Qia, Jiamin Liua, Chenpeng Lia, Weinan Hua, Siyu Tanga, Lihui Shaoa, Zhenchao Wanga,b,*( ), Guiping Ouyanga,b,c,*(
), Guiping Ouyanga,b,c,*( )
)
Received:2020-08-31
Revised:2020-11-06
Published:2020-12-10
Contact:
Zhenchao Wang, Guiping Ouyang
About author:Supported by:文章分享
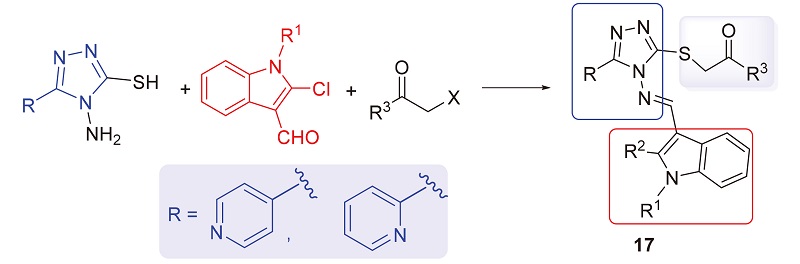
设计、合成了一系列含吡啶基的新型3-硫醚-4-吲哚亚氨基-4H-1,2,4-三唑衍生物17a~17r. 采用噻唑蓝(MTT)法对目标化合物在人类四种癌细胞A549、PC-3、HepG2、K562和大鼠正常肾细胞NRK-52E进行抗增殖活性评价. 结果显示部分化合物表现出一定的抗增殖活性, 其中乙基(E)-2-((4-(((2-氯-1-乙基-1H-吲哚-3-基)亚甲基)氨基)-5-(吡啶-4-基)-4H-1,2,4-三唑-3-基)硫代)乙酸酯(17k)对PC-3表现出较好的抗增殖活性, IC50值为9.90 μmol/L, 且对NRK-52E的细胞增殖毒性低于对照药5-氟尿嘧啶. 同时, 采用细胞迁移试验、4,6-二脒基-2-苯基吲哚(DAPI)染色、线粒体膜电位分析以及细胞凋亡、周期分析, 进一步研究了化合物 17k的作用机制, 证明化合物17k能够有效抑制肿瘤细胞的迁移, 诱发细胞凋亡, 还可以浓度依赖性方式将人前列腺癌细胞PC-3的细胞周期阻滞在G2期. 此外, 还探究了目标化合物对水稻白叶枯病菌(Xanthomonas oryzae pv. Oryzae, Xoo)的抑制活性, 初步抗菌活性结果显示: 化合物17b、17e和17h在50和100 μg/mL时, 对水稻白叶枯病菌表现出一定的抑制活性, 优于对照药叶枯唑(BMT)和噻菌铜(TDC).
漆亚云, 刘佳敏, 李成朋, 胡伟男, 唐思雨, 邵利辉, 王贞超, 欧阳贵平. 新型含吡啶基3-硫醚-4-吲哚亚氨基-4H-1,2,4-三唑衍生物的设计、合成及体外生物活性评价[J]. 有机化学, 2021, 41(4): 1670-1682.
Yayun Qi, Jiamin Liu, Chenpeng Li, Weinan Hu, Siyu Tang, Lihui Shao, Zhenchao Wang, Guiping Ouyang. Novel 3-Thioether-4-indolimino-4H-1,2,4-triazole Derivatives Bearing Pyridyl Moiety: Design, Synthesis and Bioactivity Evaluation in vitro[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1670-1682.
| Compd. | Inhibitory activitya/% at 10 μmol/L | |||
|---|---|---|---|---|
| A549 | PC-3 | HepG2 | K562 | |
| 17a | 17.02±3.33 | 27.76±5.09 | 4.60±6.71 | 5.82±2.12 |
| 17b | 17.56±5.63 | 37.13±3.89 | 8.86±5.78 | 12.52±5.33 |
| 17c | 14.89±4.85 | 40.04±4.85 | 30.28±4.85 | 27.03±4.85 |
| 17d | 22.29±1.28 | 37.63±6.32 | 9.28±5.90 | 39.34±2.53 |
| 17e | 16.13±2.55 | 27.62±3.56 | 6.03±4.71 | 11.62±2.16 |
| 17f | 18.59±6.34 | 31.38±4.78 | 5.50±6.57 | 10.64±2.69 |
| 17g | 11.11±3.22 | 25.59±2.70 | 6.52±9.90 | 13.00±5.37 |
| 17h | 14.62±2.96 | 26.71±5.83 | 11.95±4.21 | 7.79±0.83 |
| 17i | 10.04±3.34 | 18.46±6.41 | 9.15±4.67 | 14.94±5.38 |
| 17j | 10.51±6.97 | 14.22±2.86 | 14.04±1.6 | 10.06±3.57 |
| 17k | 36.90±3.46 | 32.98±2.29 | 52.80±2.40 | 29.33±1.96 |
| 17l | 36.52±3.44 | 29.74±5.89 | 21.25±6.46 | 12.10±0.37 |
| 17m | 24.16±5.60 | 36.98±4.31 | 12.65±5.27 | 16.12±5.50 |
| 17n | 12.39±4.72 | 27.96±2.90 | 11.81±5.44 | 20.57±2.98 |
| 17o | 22.99±8.21 | 40.04±7.26 | 11.30±4.48 | 14.96±7.78 |
| 17p | 23.66±8.70 | 53.40±8.89 | 11.69±5.56 | 27.18±6.34 |
| 17q | 30.59±6.66 | 36.11±1.62 | 10.52±1.38 | 18.05±1.07 |
| 17r | 48.88±5.19 | 43.30±5.54 | 9.59±5.59 | 26.88±5.39 |
| 5-Fub | 47.43±1.39 | 39.47±3.43 | 37.01±2.12 | 29.61±5.62 |
| Compd. | Inhibitory activitya/% at 10 μmol/L | |||
|---|---|---|---|---|
| A549 | PC-3 | HepG2 | K562 | |
| 17a | 17.02±3.33 | 27.76±5.09 | 4.60±6.71 | 5.82±2.12 |
| 17b | 17.56±5.63 | 37.13±3.89 | 8.86±5.78 | 12.52±5.33 |
| 17c | 14.89±4.85 | 40.04±4.85 | 30.28±4.85 | 27.03±4.85 |
| 17d | 22.29±1.28 | 37.63±6.32 | 9.28±5.90 | 39.34±2.53 |
| 17e | 16.13±2.55 | 27.62±3.56 | 6.03±4.71 | 11.62±2.16 |
| 17f | 18.59±6.34 | 31.38±4.78 | 5.50±6.57 | 10.64±2.69 |
| 17g | 11.11±3.22 | 25.59±2.70 | 6.52±9.90 | 13.00±5.37 |
| 17h | 14.62±2.96 | 26.71±5.83 | 11.95±4.21 | 7.79±0.83 |
| 17i | 10.04±3.34 | 18.46±6.41 | 9.15±4.67 | 14.94±5.38 |
| 17j | 10.51±6.97 | 14.22±2.86 | 14.04±1.6 | 10.06±3.57 |
| 17k | 36.90±3.46 | 32.98±2.29 | 52.80±2.40 | 29.33±1.96 |
| 17l | 36.52±3.44 | 29.74±5.89 | 21.25±6.46 | 12.10±0.37 |
| 17m | 24.16±5.60 | 36.98±4.31 | 12.65±5.27 | 16.12±5.50 |
| 17n | 12.39±4.72 | 27.96±2.90 | 11.81±5.44 | 20.57±2.98 |
| 17o | 22.99±8.21 | 40.04±7.26 | 11.30±4.48 | 14.96±7.78 |
| 17p | 23.66±8.70 | 53.40±8.89 | 11.69±5.56 | 27.18±6.34 |
| 17q | 30.59±6.66 | 36.11±1.62 | 10.52±1.38 | 18.05±1.07 |
| 17r | 48.88±5.19 | 43.30±5.54 | 9.59±5.59 | 26.88±5.39 |
| 5-Fub | 47.43±1.39 | 39.47±3.43 | 37.01±2.12 | 29.61±5.62 |
| Compd. | IC50a/(μmol?L-1) | |||
|---|---|---|---|---|
| A549 | PC-3 | HepG2 | K562 | |
| 17b | 25.02±6.63 | 24.97±8.11 | 43.02±7.61 | 18.32±4.41 |
| 17c | 31.77±6.66 | 12.95±0.83 | 23.12±6.70 | 11.86±3.67 |
| 17d | 15.45±1.48 | 26.31±8.86 | 17.99±1.83 | 14.39±0.65 |
| 17k | 14.22±0.60 | 9.90±3.85 | 11.36±2.72 | 13.70±0.42 |
| 17l | 16.23±3.34 | 17.54±5.00 | 22.22±0.92 | 37.23±7.64 |
| 17o | 21.78±7.08 | 31.25±8.70 | 41.15±5.11 | 22.45±3.51 |
| 17p | 29.96±3.49 | 26.60±8.06 | 17.63±8.92 | 58.49±8.25 |
| 17r | 23.11±9.21 | 16.28±3.82 | 21.37±4.49 | 28.14±3.19 |
| 5-Fub | 6.29±1.12 | 11.84±1.90 | 15.47±4.23 | 18.06±4.61 |
| Compd. | IC50a/(μmol?L-1) | |||
|---|---|---|---|---|
| A549 | PC-3 | HepG2 | K562 | |
| 17b | 25.02±6.63 | 24.97±8.11 | 43.02±7.61 | 18.32±4.41 |
| 17c | 31.77±6.66 | 12.95±0.83 | 23.12±6.70 | 11.86±3.67 |
| 17d | 15.45±1.48 | 26.31±8.86 | 17.99±1.83 | 14.39±0.65 |
| 17k | 14.22±0.60 | 9.90±3.85 | 11.36±2.72 | 13.70±0.42 |
| 17l | 16.23±3.34 | 17.54±5.00 | 22.22±0.92 | 37.23±7.64 |
| 17o | 21.78±7.08 | 31.25±8.70 | 41.15±5.11 | 22.45±3.51 |
| 17p | 29.96±3.49 | 26.60±8.06 | 17.63±8.92 | 58.49±8.25 |
| 17r | 23.11±9.21 | 16.28±3.82 | 21.37±4.49 | 28.14±3.19 |
| 5-Fub | 6.29±1.12 | 11.84±1.90 | 15.47±4.23 | 18.06±4.61 |
| Compd. | R1 | R2 | R3 | R4 | IC50a/(μmol?L–1) | |
|---|---|---|---|---|---|---|
| PC-3 | NRK-52E | |||||
| 17a | H | o-Pyridyl | H | OCH2CH3 | 23.85±5.46 | 38.78±3.56 |
| 17b | H | o-Pyridyl | H | OCH2CH2CH3 | 24.97±8.11 | 33.23±3.51 |
| 17c | H | o-Pyridyl | Cl | OCH2CH3 | 12.95±0.83 | 48.83±1.95 |
| 17d | H | o-Pyridyl | Cl | OCH2CH2CH3 | 26.31±8.86 | 33.05±2.46 |
| 17e | CH3 | o-Pyridyl | Cl | OCH2CH3 | 27.61±3.45 | 45.41±3.20 |
| 17f | CH3 | o-Pyridyl | Cl | OCH2CH2CH3 | 32.42±4.60 | 86.24±3.46 |
| 17g | CH2CH3 | o-Pyridyl | Cl | OCH2CH3 | 16.74±2.53 | 81.96±0.77 |
| 17h | CH2CH3 | o-Pyridyl | Cl | OCH2CH2CH3 | 44.31±1.14 | 49.34±3.76 |
| 17i | CH2CH2CH3 | o-Pyridyl | Cl | OCH2CH3 | 31.25±1.75 | 36.68±3.20 |
| 17j | CH2CH2CH3 | o-Pyridyl | Cl | OCH2CH2CH3 | 33.15±2.35 | 44.55±3.87 |
| 17k | CH2CH3 | p-Pyridyl | Cl | OCH2CH3 | 9.90±3.85 | 51.73±4.48 |
| 17l | CH2CH3 | p-Pyridyl | Cl | Ph | 17.54±5.00 | 19.83±3.00 |
| 17m | H | p-Pyridyl | H | OCH2CH3 | 23.28±1.84 | 65.97±1.95 |
| 17n | H | p-Pyridyl | Cl | OCH2CH3 | 12.58±4.26 | 69.19±2.55 |
| 17o | H | p-Pyridyl | Cl | OCH2CH2CH3 | 31.25±8.70 | 61.21±5.02 |
| 17p | CH3 | p-Pyridyl | Cl | OCH2CH2CH3 | 26.60±8.06 | 50.77±4.57 |
| 17q | CH2CH2CH3 | p-Pyridyl | Cl | OCH2CH3 | 15.50±1.42 | 32.91±5.22 |
| 17r | CH2CH2CH3 | p-Pyridyl | Cl | OCH2CH2CH3 | 16.28±3.82 | 27.60±4.80 |
| 5-Fub | — | 11.84±1.90 | 14.13±1.34 | |||
| Compd. | R1 | R2 | R3 | R4 | IC50a/(μmol?L–1) | |
|---|---|---|---|---|---|---|
| PC-3 | NRK-52E | |||||
| 17a | H | o-Pyridyl | H | OCH2CH3 | 23.85±5.46 | 38.78±3.56 |
| 17b | H | o-Pyridyl | H | OCH2CH2CH3 | 24.97±8.11 | 33.23±3.51 |
| 17c | H | o-Pyridyl | Cl | OCH2CH3 | 12.95±0.83 | 48.83±1.95 |
| 17d | H | o-Pyridyl | Cl | OCH2CH2CH3 | 26.31±8.86 | 33.05±2.46 |
| 17e | CH3 | o-Pyridyl | Cl | OCH2CH3 | 27.61±3.45 | 45.41±3.20 |
| 17f | CH3 | o-Pyridyl | Cl | OCH2CH2CH3 | 32.42±4.60 | 86.24±3.46 |
| 17g | CH2CH3 | o-Pyridyl | Cl | OCH2CH3 | 16.74±2.53 | 81.96±0.77 |
| 17h | CH2CH3 | o-Pyridyl | Cl | OCH2CH2CH3 | 44.31±1.14 | 49.34±3.76 |
| 17i | CH2CH2CH3 | o-Pyridyl | Cl | OCH2CH3 | 31.25±1.75 | 36.68±3.20 |
| 17j | CH2CH2CH3 | o-Pyridyl | Cl | OCH2CH2CH3 | 33.15±2.35 | 44.55±3.87 |
| 17k | CH2CH3 | p-Pyridyl | Cl | OCH2CH3 | 9.90±3.85 | 51.73±4.48 |
| 17l | CH2CH3 | p-Pyridyl | Cl | Ph | 17.54±5.00 | 19.83±3.00 |
| 17m | H | p-Pyridyl | H | OCH2CH3 | 23.28±1.84 | 65.97±1.95 |
| 17n | H | p-Pyridyl | Cl | OCH2CH3 | 12.58±4.26 | 69.19±2.55 |
| 17o | H | p-Pyridyl | Cl | OCH2CH2CH3 | 31.25±8.70 | 61.21±5.02 |
| 17p | CH3 | p-Pyridyl | Cl | OCH2CH2CH3 | 26.60±8.06 | 50.77±4.57 |
| 17q | CH2CH2CH3 | p-Pyridyl | Cl | OCH2CH3 | 15.50±1.42 | 32.91±5.22 |
| 17r | CH2CH2CH3 | p-Pyridyl | Cl | OCH2CH2CH3 | 16.28±3.82 | 27.60±4.80 |
| 5-Fub | — | 11.84±1.90 | 14.13±1.34 | |||
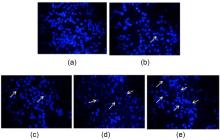
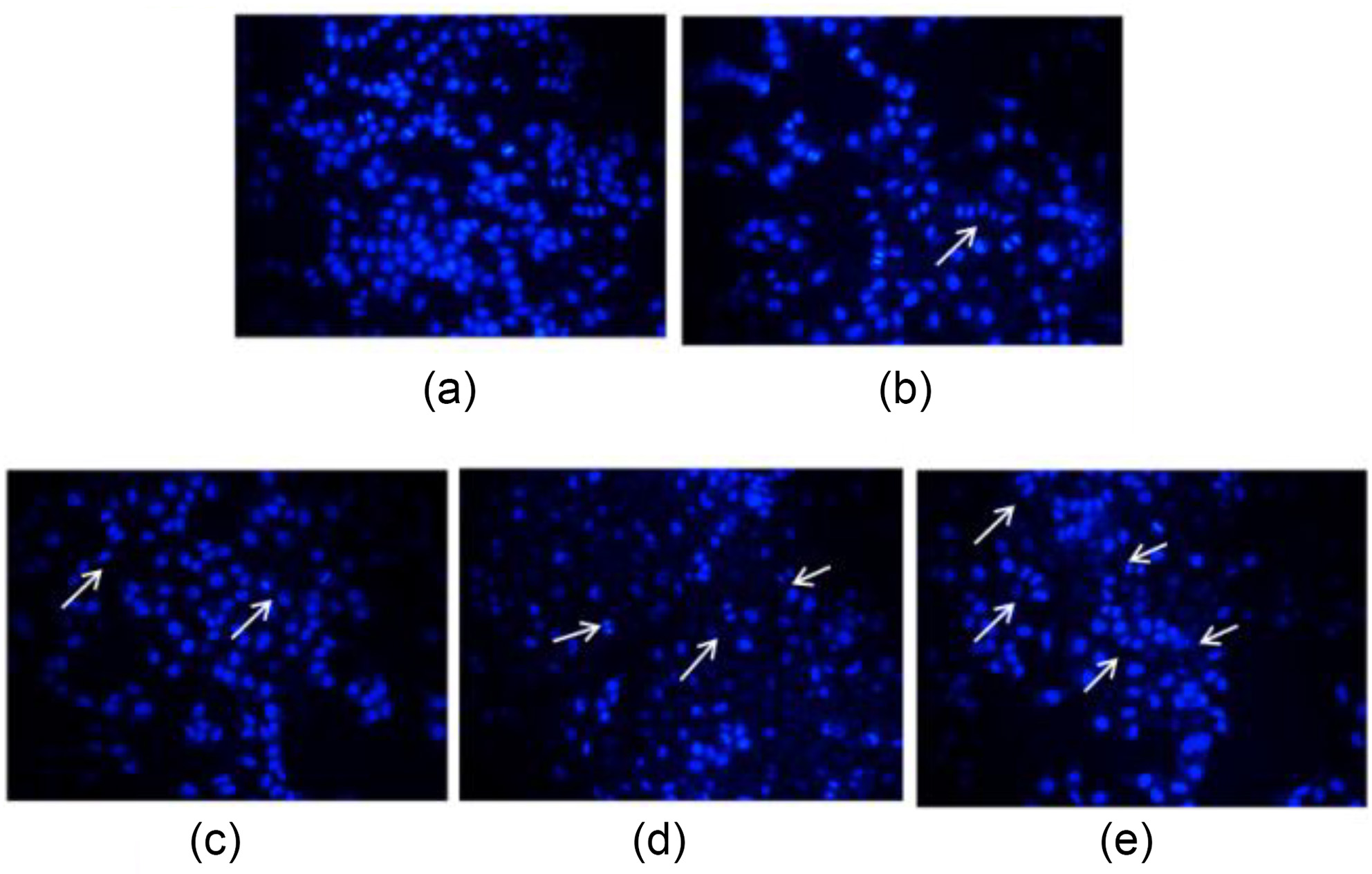


| Compd. | Xoo | Compd. | Xoo | ||
|---|---|---|---|---|---|
| 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | ||
| 17a | 36.96±0.66 | 60.69±5.54 | 17k | 25.81±8.25 | 48.86±6.25 |
| 17b | 43.01±6.57 | 66.43±9.54 | 17l | 23.14±3.46 | 68.88±4.74 |
| 17c | 28.81±4.92 | 63.33±1.10 | 17m | 15.02±1.45 | 42.02±2.46 |
| 17d | 27.18±4.25 | 57.75±2.06 | 17n | 6.87±2.59 | 39.38±5.37 |
| 17e | 44.17±3.61 | 67.01±2.93 | 17o | 23.18±3.56 | 54.17±7.77 |
| 17f | 26.02±9.02 | 59.91±8.83 | 17p | 22.02±8.37 | 56.33±6.42 |
| 17g | 27.55±1.31 | 65.17±2.47 | 17q | 21.49±5.22 | 58.54±7.50 |
| 17h | 35.83±4.81 | 71.27±1.58 | 17r | 29.97±4.84 | 54.70±6.76 |
| 17i | 21.49±5.29 | 53.96±2.83 | BMTb | 28.02±4.49 | 64.38±0.86 |
| 17j | 25.39±2.77 | 47.33±9.09 | TDCb | 32.65±7.54 | 53.96±0.71 |
| Compd. | Xoo | Compd. | Xoo | ||
|---|---|---|---|---|---|
| 50 μg/mL | 100 μg/mL | 50 μg/mL | 100 μg/mL | ||
| 17a | 36.96±0.66 | 60.69±5.54 | 17k | 25.81±8.25 | 48.86±6.25 |
| 17b | 43.01±6.57 | 66.43±9.54 | 17l | 23.14±3.46 | 68.88±4.74 |
| 17c | 28.81±4.92 | 63.33±1.10 | 17m | 15.02±1.45 | 42.02±2.46 |
| 17d | 27.18±4.25 | 57.75±2.06 | 17n | 6.87±2.59 | 39.38±5.37 |
| 17e | 44.17±3.61 | 67.01±2.93 | 17o | 23.18±3.56 | 54.17±7.77 |
| 17f | 26.02±9.02 | 59.91±8.83 | 17p | 22.02±8.37 | 56.33±6.42 |
| 17g | 27.55±1.31 | 65.17±2.47 | 17q | 21.49±5.22 | 58.54±7.50 |
| 17h | 35.83±4.81 | 71.27±1.58 | 17r | 29.97±4.84 | 54.70±6.76 |
| 17i | 21.49±5.29 | 53.96±2.83 | BMTb | 28.02±4.49 | 64.38±0.86 |
| 17j | 25.39±2.77 | 47.33±9.09 | TDCb | 32.65±7.54 | 53.96±0.71 |
| [1] |
Chabner, B.A.; Roberts, T.G. Nat. Rev. Cancer 2005, 5,65.
|
| [2] |
Aytac, P.S.; Durmaz, I.; Houston, D.R.; Cetin-Atalay, R.; Tozkoparan, B. Bioorg. Med. Chem. 2016, 24,858.
|
| [3] |
Kulabas, N.; Tatar, E.; Ozakpınar, O.B.; Ozsavci, D.; Pannecouque, C.; Clercq, E.D.; Küçükgüzel, I. Eur. J. Med. Chem. 2016, 121,58.
|
| [4] |
El-Sherief, H.A. M.; Youssif, B.G. M.; Abbas Bukhari, S.N.; Abdelazeem, A.H.; Abdel-Aziz, M.; Abdel-Rahman, H.M. Eur. J. Med. Chem. 2018, 156,774.
|
| [5] |
Boraei, A.T. A.; Singh, P.K.; Sechi, M.; Satta, S. Eur. J. Med. Chem. 2019, 182,111621.
pmid: 31442685 |
| [6] |
Aboeldahab, A.M. A.; Beshr, E.A. M.; Shoman, M.E.; Aly, O.M. Eur. J. Med. Chem. 2018, 146,79.
|
| [7] |
Khan, I.; Zaib, S.; Ibrar, A.; Ahmad, S.; Furtmann, N.; Hameed, S.; Simpson, J.; Bajorath, J.; Iqbal, J. Eur. J. Med. Chem. 2014, 78,167.
pmid: 24681981 |
| [8] |
Kamel, M.M.; Abdo, N.Y. M. Eur. J. Med. Chem. 2014, 86,75.
|
| [9] |
Sekhar, M.M.; Nagarjuna, U.; PadmavathiJ, V.; Padmaja, A.; Vasudeva Reddy, N.; Vijaya, T. Eur. J. Med. Chem, 2018, 145,1.
|
| [10] |
Fan, Y.Ke.; L., X.; Li, M. J. Heterocycl. Chem. 2018, 55,791.
|
| [11] |
Almajan, G.L.; Barbuceanu, S.F.; Saramet, I.; Draghici, C.; Eur. J. Med. Chem. 2010, 45,3191.
|
| [12] |
Aouad, M.R.; Mayaba, M.M.; Naqvi, A.; Bardaweel, S.K.; Al-blewi, F.F.; Messali, M.; Rezki, N. Chem. Cent. J. 2017, 11,117.
pmid: 29159721 |
| [13] |
Prakash, O.; Aneja, D.K.; Hussai, K.; Lohan, P.; Ranjan, P.; Arora, S.; Sharmac, C.; Aneja, K,P. Eur. J. Med. Chem. 2011, 46,5065.
|
| [14] |
Sarigol, D.; Uzgoren-Baran, A.; Tel, B.C.; Somuncuoglu, E.I.; Kazkayas, I.; Ozadali-Sari, K.; Unsal-Tan, O.; Okay, G.; Ertan, M.; Tozkoparan, B. Bioorg. Med. Chem. 2015, 23,2518.
|
| [15] |
Sumangala, V.; Poojary, B.; Chidananda, N.; Arulmoli, T.; Shenoy, S. Med. Chem. Res. 2013, 22,2921.
|
| [16] |
Ahirwar, J.; Ahirwar, D.; Lanjhiyana, S.; Jha, A.K.; Dewangan, D.; Badwaik, H. J. Heterocycl. Chem. 2018, 55,2130.
|
| [17] |
Wang, B.L.; Shi, Y.X.; Ma, Y.; Liu, X.H.; Li, Y.H.; Song, H.B.; Li, Z.M. J. Agric. Food Chem. 2010, 58,5515.
pmid: 20384340 |
| [18] |
Fan, C.C.; Jiao, S.L.; Qin, M.; Zou, Z.H. ChemistrySelect 2019, 4,8593.
|
| [19] |
Aly, A.A. A.; Hassan, A.; Makhlouf, M.M.; Bräse, S. Molecules 2020, 25.3036.
|
| [20] |
Cheng, Y.N.; Jiang, Z.H.; Sun, L.S. Eur. J. Med. Chem., 2020, 200,112463.
|
| [21] |
Demirbas, N.; Demirbas, A.; Karaoglu, S.A.; Celik, E. ARKIVOC 2005,75.
|
| [22] |
Tian, K.; Meng, J.; Gan, Y.Y.; Li, X.Q.; Wu, S.Q.; Chen, J.; Qi, Y.Y.; Hu, W.N.; Wang, Z.C.; OuYang, G.P. Chin. J. Org. Chem. 2018, 38,2657. (in Chinese)
|
|
( 田坤, 孟娇, 甘宜远, 李小琴, 巫受群, 陈洁, 漆亚云, 胡伟男, 王贞超, 欧阳贵平, 有机化学, 2018, 38,2657.)
|
|
| [23] |
Li, W.; Qi, Y.Y.; Wang, Y.Y.; Gan, Y.Y.; Shao, L.H.; Zhang, L.Q.; Tang, Z.H.; Zhu, M.; Tang, S.Y.; Wang, Z.C.; OuYang, G.P. J. Heterocycl. Chem. 2020, 57,2548.
|
| [24] |
Li, X.Q.; Gan, Y.Y.; Meng, J.; Li, W.; Chen, J. Qi, Y.Y.; Tian, K.; OuYang, G.P.; Wang, Z.C. J. Heterocycl. Chem. 2018, 55,1382.
|
| [25] |
Tian, K.; Li, X.Q.; Zhang, L.; Gan, Y.Y.; Wu, S.Q.; Wan, J.L.; Xu, Y.; Cai, C.T.; OuYang, G.P.; Wang, Z.C. Chem. Pap. 2019, 73,17.
|
| [26] |
Hassan, A.Y.; Sarg, M.T.; El‐Sebaey, S.A. J. Heterocycl. Chem. 2020, 57,694.
|
| [27] |
Parameshwarappa, G.; Lingamani, J.; Patil, S.B.; Goudgaon, A.M. Heterocycl. Commun. 2009, 15,343.
|
| [28] |
Li, Q.; Wang, Y.; Hu, M.J.; Chen, P.; You, W.W.; Zhao, P. Chin. J. Org. Chem. 2017, 37,967. (in Chinese)
|
|
( 黎秋, 汪雨, 陈鹏, 游文玮, 赵培亮, 有机化学, 2017, 37,967.)
|
|
| [29] |
Das Mukherjee, D.; Kumar, N.M.; Tantak, M.P.; Das, A.; Ganguli, A.; Datta, S.; Kumar, D.; Chakrabarti, G. Biochemistry 2016, 55,3020.
pmid: 27110637 |
| [30] |
Wu, S.Q.; Li, X.Q.; Meng, J. Gan, Y.Y.; Tian, K.; Wang, Z.C.; OuYang, G.P. Chin. J. Org. Chem. 2018, 38,1447. (in Chinese)
|
|
( 巫受群, 李小琴, 孟娇, 甘宜远, 田坤, 王贞超, 欧阳贵平, 有机化学, 2018, 38,1447.)
|
|
| [31] |
Zhang, B.; Li, Y.H.; Liu, Y.; Chen, Y.R.; Pan, E.S.; You, W.W.; Zhao, P.L. Eur. J. Med. Chem. 2015, 103,335.
pmid: 26363869 |
| [1] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [2] | 李鹏辉, 谢青洋, 万福贤, 张元红, 姜林. 含环丙基的新型取代嘧啶-5-甲酰胺的合成及杀菌活性研究[J]. 有机化学, 2024, 44(2): 650-656. |
| [3] | 王博珍, 张婕, 粘春惠, 金茗茗, 孔苗苗, 李物兰, 何文斐, 吴建章. 含有3,4-二氯苯基的酰胺类化合物的合成及抗肿瘤活性研究[J]. 有机化学, 2024, 44(1): 232-241. |
| [4] | 岁丹丹, 岑南楠, 龚若蕖, 陈阳, 陈文博. 无支持电解质条件下连续流电化学合成三氟甲基化氧化吲哚[J]. 有机化学, 2023, 43(9): 3239-3245. |
| [5] | 贝文峰, 潘健, 冉冬梅, 刘伊琳, 杨震, 冯若昆. 基于钴催化吲哚酰胺与二炔和单炔的[4+2]环化反应合成γ-咔啉酮[J]. 有机化学, 2023, 43(9): 3226-3238. |
| [6] | 冯莹珂, 王贺, 崔梦行, 孙然, 王欣, 陈阳, 李蕾. 可见光诱导的新型官能化芳基异腈化合物的二氟烷基化环化反应[J]. 有机化学, 2023, 43(8): 2913-2925. |
| [7] | 张彦波, 孙萌. 铑催化碳酸亚乙烯酯与吲哚啉C(7)位C—H甲酰甲基化反应[J]. 有机化学, 2023, 43(8): 2905-2912. |
| [8] | 王熠, 张键, 刘飏子, 罗晓燕, 邓卫平. 钯催化不对称[3+4]环加成构建吲哚并环庚烷[J]. 有机化学, 2023, 43(8): 2864-2877. |
| [9] | 张维舒, 聂礼飞, Khurshed Bozorov, 阿吉艾克拜尔•艾萨, 赵江瑜. 2,5-二氨基噻吩-3,4-二羧酸二乙酯衍生物的合成及抗肿瘤活性研究[J]. 有机化学, 2023, 43(7): 2543-2552. |
| [10] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [11] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [12] | 孙泽人, 翟冰新, 何光超, 沈慧, 陈琳雅, 张杉, 邹毅, 朱启华, 徐云根. 新型1,2,3-三氮唑类衍生物的合成及抗炎活性研究[J]. 有机化学, 2023, 43(6): 2143-2155. |
| [13] | 庞盼杏, 宁蓉, 祝创, 黄文洁, 马献力, 蒋彩娜, 李芳耀, 周小群. 苦参碱缩氨基脲类化合物的合成及其体外抗肿瘤活性研究[J]. 有机化学, 2023, 43(6): 2126-2135. |
| [14] | 庞明杨, 常宏宏, 冯璋, 张娟. 过渡金属催化吲哚的串联去芳构化反应研究进展[J]. 有机化学, 2023, 43(4): 1271-1291. |
| [15] | 黄华, 李鑫, 苏建科, 宋秋玲. 二氟卡宾参与下从邻乙烯基苯胺出发构建3-取代吲哚酮类化合物[J]. 有机化学, 2023, 43(3): 1146-1156. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||