有机化学 ›› 2021, Vol. 41 ›› Issue (4): 1683-1690.DOI: 10.6023/cjoc202009044 上一篇 下一篇
所属专题: 有机光催化虚拟合辑
研究论文
收稿日期:2020-09-20
修回日期:2020-11-15
发布日期:2020-12-10
通讯作者:
左伟伟
基金资助:
Shangfei Huo1, Hong Chen1, Weiwei Zuo1,*( )
)
Received:2020-09-20
Revised:2020-11-15
Published:2020-12-10
Contact:
Weiwei Zuo
About author:Supported by:文章分享
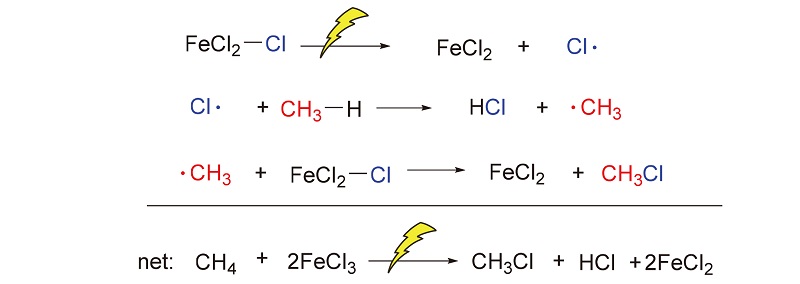
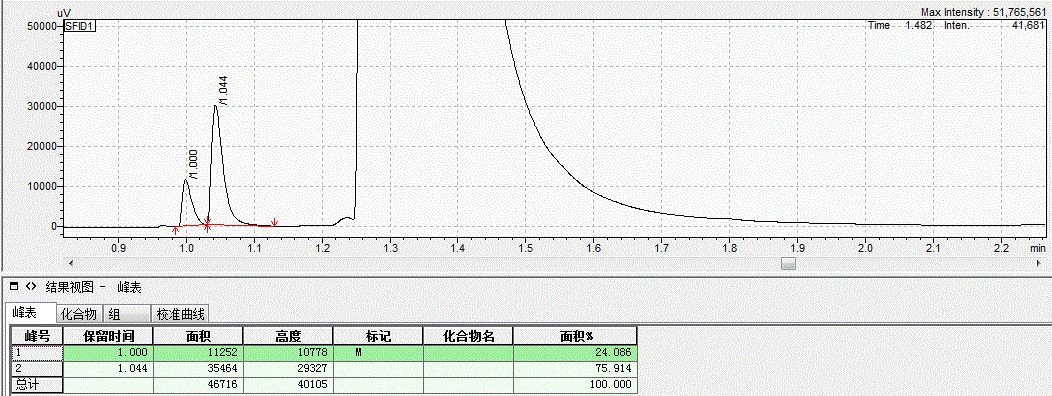
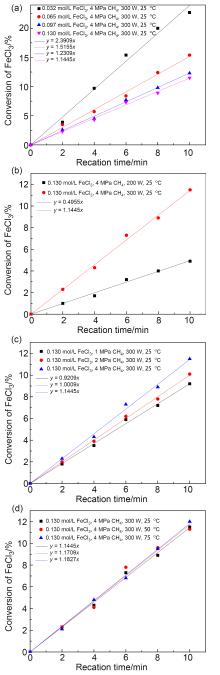
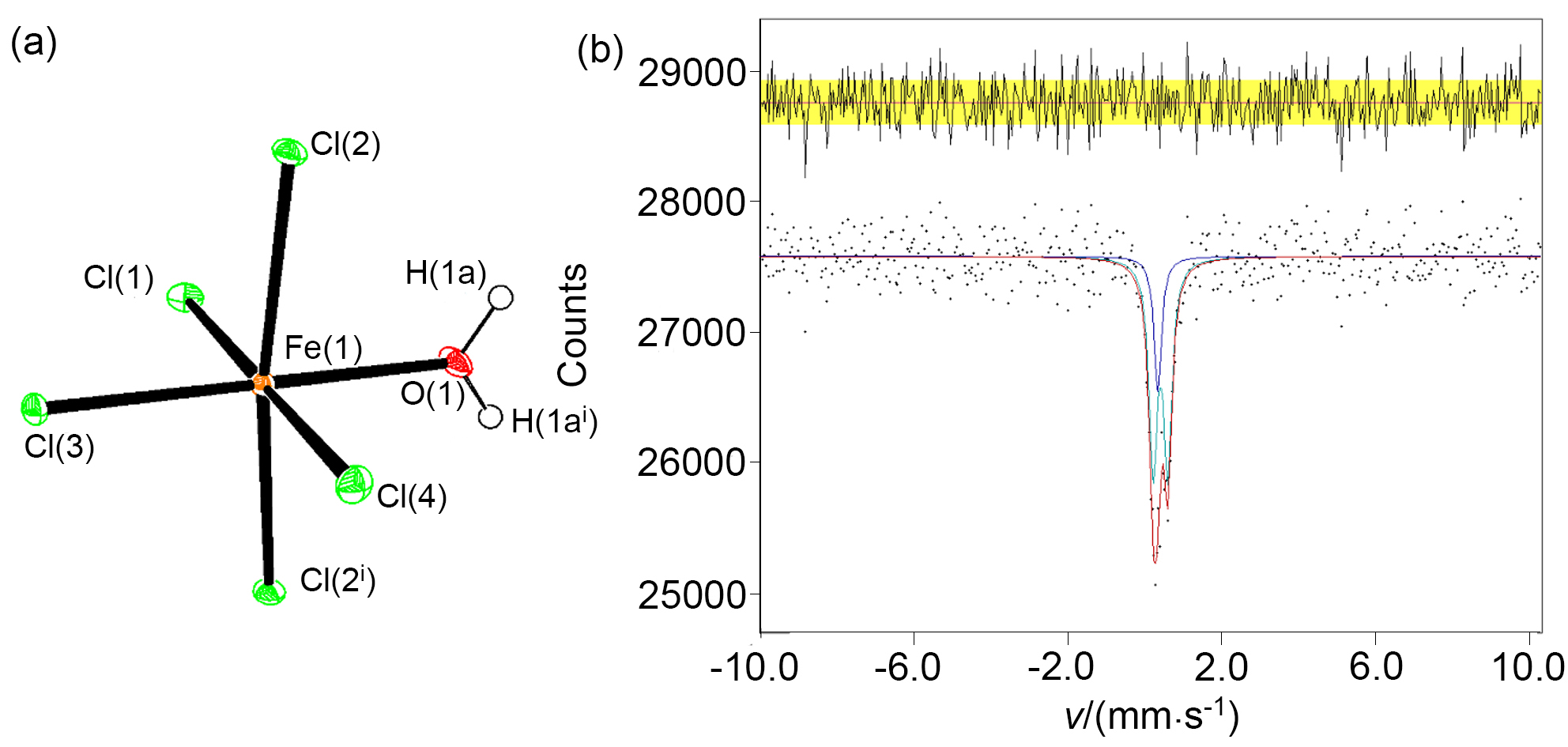
在温和条件下选择性活化与转化甲烷具有可观的经济效益, 但同时也是难点. 报道在环境温度条件下, 无水三氯化铁(FeCl3)与甲烷在环境温度下的光化学反应, 这是一种有效的选择性活化甲烷生成一氯甲烷的方法. 在300 W的高压汞灯或者太阳光的照射下, 由甲烷转化的产物为单一的一氯甲烷, 而没有其它多氯代物, 最大初始生成效率为43 gMeCl?(kgFe?h)–1. 氯化铁既作为氯自由基源, 也作为氧化剂, 并在反应中被还原为氯化亚铁. 高压汞灯功率增加以及氯化铁浓度增加都会加快初始反应速率. 而反应温度以及甲烷的压力对反应的初始速率影响很小. 氯甲烷可以被水解为甲醇, 亚铁在空气以及盐酸存在下也可以被重新氧化为三价铁, 从而完成铁和氯的循环.
霍尚飞, 陈鸿, 左伟伟. 甲烷与三氯化铁的光化学反应在环境温度下实现甲烷的选择性氯化反应[J]. 有机化学, 2021, 41(4): 1683-1690.
Shangfei Huo, Hong Chen, Weiwei Zuo. Selective Chlorination of Methane Photochemically Mediated by Ferric Chloride at Ambient Temperature[J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1683-1690.





| [1] |
Lamarche-Gagnon, G.; Wadham, J.L.; Sherwood Lollar, B.; Arndt, S.; Fietzek, P.; Beaton, A.D.; Tedstone, A.J.; Telling, J.; Bagshaw, E.A.; Hawkings, J.R.; Kohler, T.J.; Zarsky, J.D.; Mowlem, M.C.; Anesio, A.M.; Stibal, M. Nature 2019, 565,73.
doi: 10.1038/s41586-018-0800-0 pmid: 30602750 |
| [2] |
(a) McFarland, E. Science 2012, 338,340.
pmid: 28150944 |
|
(b) Gunsalus, N.J.; Koppaka, A.; Park, S.H.; Bischof, S.M.; Hashiguchi, B.G.; Periana, R.A. Chem. Rev. 2017, 117,8521.
doi: 10.1021/acs.chemrev.6b00739 pmid: 28150944 |
|
|
(c) Lin, R.; Amrute, A.P.; Perez-Ramirez, J. Chem. Rev. 2017, 117,4182.
doi: 10.1021/acs.chemrev.6b00551 pmid: 28150944 |
|
|
(d) Ravi, M.; Ranocchiari, M.; van Bokhoven, J.A. Angew. Chem., nt. Ed. 2017, 56,16464.
pmid: 28150944 |
|
| [3] |
(a) Fletcher, S.E. M.; Schaefer, H. Science 2019, 364,932.
doi: 10.1126/science.aax1828 pmid: 12037558 |
|
(b) Labinger, J.A.; Bercaw, J.E. Nature 2002, 417,507.
pmid: 12037558 |
|
|
(c) Shilov, A.E.; Shul'pin, G.B. Chem. Rev. 1997, 97,2879.
pmid: 12037558 |
|
|
(d) Cavaliere, V.N.; Wicker, B.F.; Mindiola, D.J. Adv. Organomet. Chem. 2012, 60,1.
pmid: 12037558 |
|
| [4] |
(a) Alvarez-Galvan, M.C.; Mota, N.; Ojeda, M.; Rojas, S.; Navarro, R.M.; Fierro, J.L. G. Catal. Today 2011, 171,15.
|
|
(b) Olsbye, U. Angew. Chem., nt. Ed. 2016, 55,7294.
|
|
| [5] |
(a) Zimmermann, T.; Soorholtz, M.; Bilke, M.; Schuth, F. J. Am. Chem. Soc. 2016, 138,12395.
doi: 10.1021/jacs.6b05167 pmid: 9554841 |
|
(b) Periana, R.A.; Taube, D.J.; Evitt, E.R.; Loffler, D.G.; Wentrcek, P.R.; Voss, G.; Masuda, T. Science 1993, 259,340.
pmid: 9554841 |
|
|
(c) Periana, R.A.; Taube, D.J.; Gamble, S.; Taube, H.; Satoh, T.; Fujii, H. Science 1998, 280,560.
doi: 10.1126/science.280.5363.560 pmid: 9554841 |
|
|
(d) Lin, M.; Sen, A. Nature 1994, 368,613.
pmid: 9554841 |
|
|
(e) Periana, R.A.; Mironov, O.; Taube, D.; Bhalla, G.; Jones, C.J. Science 2003, 301,814.
pmid: 9554841 |
|
|
(f) Gretz, E.; Oliver, T.F.; Sen, A. J. Am. Chem. Soc. 1987, 109,8109.
pmid: 9554841 |
|
|
(g) Vargaftik, M.N.; Stolarov, I.P.; Moiseev, I.I. J. Chem. Soc., hem. Commun. 1990,1049.
pmid: 9554841 |
|
|
(h) Kao, L.C.; Hutson, A.C.; Sen, A. J. Am. Chem. Soc. 1991, 113,700.
pmid: 9554841 |
|
| [6] |
(a) Takanabe, K.; Iglesia, E. Angew. Chem., nt. Ed. 2008, 47,7689.
pmid: 30924338 |
|
(b) Hammond, C.; Conrad, S.; Hermans, I. ChemSusChem 2012, 5,1668.
pmid: 30924338 |
|
|
(c) Lunsford, J.H. Angew. Chem., nt. Ed. Engl. 1995, 34,970.
pmid: 30924338 |
|
|
(d) Meng, L.; Chen, Z.; Ma, Z.; He, S.; Hou, Y.; Li, H.-H.; Yuan, R.; Huang, X.-H.; Wang, X.; Wang, X.; Long, J. Energy Environ. Sci. 2018, 11,294.
pmid: 30924338 |
|
|
(e) Wu, S.; Tan, X.; Lei, J.; Chen, H.; Wang, L.; Zhang, J. J. Am. Chem. Soc. 2019, 141,6592.
pmid: 30924338 |
|
| [7] |
Horn, R.; Schloegl, R. Catal. Lett. 2015, 145,23.
|
| [8] |
(a) Bilke, M.; Losch, P.; Vozniuk, O.; Bodach, A.; Schuth, F. J. Am. Chem. Soc. 2019, 141,11212.
doi: 10.1021/jacs.9b04413 pmid: 17295483 |
|
(b) Olah, G.A.; Gupta, B.; Farina, M.; Felberg, J.D.; Ip, W.M.; Husain, A.; Karpeles, R.; Lammertsma, K.; Melhotra, A.K.; Trivedi, N.J. J. Am. Chem. Soc. 1985, 107,7097.
pmid: 17295483 |
|
|
(c) Zhou, X.P.; Yilmaz, A.; Yilmaz, G.A.; Lorkovic, I.M.; Laverman, L.E.; Weiss, M.; Sherman, J.H.; McFarland, E.W.; Stucky, G.D.; Ford, P.C. Chem. Commun. 2003,2294.
pmid: 17295483 |
|
|
(d) Paunovic, V.; Zichittella, G.; Moser, M.; Amrute, A.P.; Perez-Ramirez, J. Nat. Chem. 2016, 8,803.
doi: 10.1038/nchem.2522 pmid: 17295483 |
|
|
(e) Podkolzin, S.G.; Stangland, E.E.; Jones, M.E.; Peringer, E.; Lercher, J.A. J. Am. Chem. Soc. 2007, 129,2569.
pmid: 17295483 |
|
|
(f) He, J.; Xu, T.; Wang, Z.; Zhang, Q.; Deng, W.; Wang, Y. Angew. Chem., nt. Ed. 2012, 51,2438.
pmid: 17295483 |
|
| [9] |
(a) Li, F.; Yuan, G. Angew. Chem., nt. Ed. 2006, 45,6541.
pmid: 29199824 |
|
(b) Batamack, P.T. D.; Mathew, T.; Prakash, G.K. S. J. Am. Chem. Soc. 2017, 139,18078.
doi: 10.1021/jacs.7b10725 pmid: 29199824 |
|
| [10] |
(a) Paunovic, V.; Lin, R.; Scharfe, M.; Amrute, A.P.; Mitchell, S.; Hauert, R.; Perez-Ramirez, J. Angew. Chem., nt. Ed. 2017, 56,9791.
|
|
(b) Shalygin, A.; Paukshtis, E.; Kovalyov, E.; Bal'Zhinimaev, B. Front. Chem. Sci. Eng. 2013, 7,279.
|
|
| [11] |
(a) Maldotti, A.; Molinari, A.; Amadelli, R. Chem. Rev. 2002, 102,3811.
doi: 10.1021/cr010364p pmid: 12371903 |
|
(b) Wu, W.; Fu, Z.; Wen, X.; Wang, Y.; Zou, S.; Meng, Y.; Liu, Y.; Kirk, S.R.; Yin, D. Appl. Catal. A: Gen. 2014, 469,483.
pmid: 12371903 |
|
| [12] |
Blanksby, S.J.; Ellison, G.B. Acc. Chem. Res. 2003, 36,255.
pmid: 12693923 |
| [13] |
(a) Minisci, F.; Fontana, F. Tetrahedron Lett. 1994, 35,1427.
pmid: 17737550 |
|
(b) Kochi, J.K. Science 1967, 155,415.
doi: 10.1126/science.155.3761.415 pmid: 17737550 |
|
| [14] |
Bach, R.D.; Shobe, D.S.; Schlegel, H.B.; Nagel, C.J. J. Phys. Chem. 1996, 100,8770.
|
| [15] |
Chang, P. K. A.J.-H. C. J. Org. Chem. 1971, 36,3138.
|
| [16] |
Miller, J. WO 9959946, 1999.
|
| [17] |
(a) Roberts, B.P. Chem. Soc. Rev. 1999, 28,25.
pmid: 28636596 |
|
(b) Le, C.; Liang, Y.; Evans, R.W.; Li, X.; Mac-Millan, D.W. C. Nature 2017, 547,79.
pmid: 28636596 |
|
| [18] |
(a) Sorokin, A.B.; Kudrik, E.V.; Alvarez, L.X.; Afanasiev, P.; Millet, J.M. M.; Bouchu, D. Catal. Today 2010, 157,149.
pmid: 17676842 |
|
(b) An, Z.-J.; Pan, X.-L.; Liu, X.-M.; Han, X.-W.; Bao, X.-H. J. Am. Chem. Soc. 2006, 128,16028.
pmid: 17676842 |
|
|
(c) Bar-Nahum, I.; Khenkin, A.M.; Neumann, R. J. Am. Chem. Soc. 2004, 126,10236.
doi: 10.1021/ja0493547 pmid: 17676842 |
|
|
(d) Muehlhofer, M.; Strassner, T.; Herrmann, W.A. Angew. Chem., nt. Ed. 2002, 41,1745.
pmid: 17676842 |
|
|
(e) Kirillova, M.V.; Kuznetsov, M.L.; Reis, P.M.; da Silva, J.A. L.; da Silva, J. J., R.F.; Pombeiro, A.J. L. J. Am. Chem. Soc. 2007, 129,10531.
doi: 10.1021/ja072531u pmid: 17676842 |
|
| [19] |
(a) Lange, J.-P. ChemSusChem 2017, 10,245.
doi: 10.1002/cssc.201600855 pmid: 27763723 |
|
(b) Lange, J.P. CATTECH 2001, 5,82.
pmid: 27763723 |
|
| [20] |
Lange, J.-P. Catal. Sci. Technol. 2016, 6,4759.
|
| [21] |
Lange, J.-P.; Sushkevich, V.L.; Knorpp, A.J.; van Bokhoven, J.A. Ind. Eng. Chem. Res. 2019, 58,8674.
|
| [22] |
Knuuttila, P.T.; Jokinen, S.S.; Judin, V.O.; Vuorisalo, J.T.; Salanne, S.S. EP 0493023, 1992.
|
| [23] |
Horvath, I.T.; Pa, N.H.; Summit, J.M. M.; Cook, R.A. US 5354916, 1994.
|
| [24] |
Mizuta, S.; Kondo, W.; Fujii, K.; Iida, H.; Isshiki, S.; Noguchi, H.; Kikuchi, T.; Sue, H.; Sakai, K. Ind. Eng. Chem. Res. 1991, 30,1601.
|
| [1] | 刘继宇, 李圣玉, 陈款, 朱茵, 张元. 三苯胺功能化有序介孔聚合物作为无金属光催化剂用于二硫化物合成[J]. 有机化学, 2024, 44(2): 605-612. |
| [2] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [3] | 朱彦硕, 王红言, 舒朋华, 张克娜, 王琪琳. 烷氧自由基引发1,5-氢原子转移实现C(sp3)—H键官能团化的研究进展[J]. 有机化学, 2024, 44(1): 1-17. |
| [4] | 童红恩, 郭宏宇, 周荣. 可见光促进惰性碳-氢键对羰基的加成反应进展[J]. 有机化学, 2024, 44(1): 54-69. |
| [5] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [6] | 赵红琼, 于淼, 宋冬雪, 贾琦, 刘颖杰, 季宇彬, 许颖. 羧酸脱羧羟基化反应研究进展[J]. 有机化学, 2024, 44(1): 70-84. |
| [7] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [8] | 刘颖杰, 石岗庆, 仇格, 张鑫, 宋冬雪, 陈宁, 于淼, 许颖. 光/电催化醚α-位官能团化研究进展[J]. 有机化学, 2023, 43(8): 2664-2681. |
| [9] | 杨晓娜, 郭宏宇, 周荣. 可见光促进有机硅化合物参与的化学转化[J]. 有机化学, 2023, 43(8): 2720-2742. |
| [10] | 普佳霞, 贾小英, 韩丽荣, 李清寒. 可见光诱导C—N键断裂构建C—C键的研究进展[J]. 有机化学, 2023, 43(8): 2591-2613. |
| [11] | 王灵娜, 刘晓庆, 林钢, 金泓颖, 焦民均, 刘雪粉, 罗书平. 光促进双(4-二苯甲酮)苯醚催化C(sp3)—H键活化构建C—S键[J]. 有机化学, 2023, 43(8): 2848-2854. |
| [12] | 赵瑜, 张凯, 白育斌, 张琰图, 史时辉. 无金属条件下可见光催化与溴盐协同促进烯烃的氢硅化反应研究[J]. 有机化学, 2023, 43(8): 2837-2847. |
| [13] | 刘亚鑫, 张渔, 罗书平. 热延迟荧光(TADF)光敏剂的设计合成及其光催化脱卤反应性能研究[J]. 有机化学, 2023, 43(7): 2476-2483. |
| [14] | 陈宁, 张成栋, 李鹏, 仇格, 刘颖杰, 张天雷. 光/电化学驱动螺环化合物的合成研究进展[J]. 有机化学, 2023, 43(7): 2293-2303. |
| [15] | 徐忠荣, 万结平, 刘云云. 基于热、光以及电化学过程的无过渡金属碳-氢键硫氰化和硒氰化反应[J]. 有机化学, 2023, 43(7): 2425-2446. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||