有机化学 ›› 2025, Vol. 45 ›› Issue (8): 2836-2847.DOI: 10.6023/cjoc202503014 上一篇 下一篇
研究论文
赵玲, 朱小慧, 陈华, 郑学丽, 薛卫超, 徐嘉麒, 付海燕*( ), 李瑞祥*(
), 李瑞祥*( )
)
收稿日期:2025-03-14
修回日期:2025-04-14
发布日期:2025-04-24
基金资助:
Ling Zhao, Xiaohui Zhu, Hua Chen, Xueli Zheng, Weichao Xue, Jiaqi Xu, Haiyan Fu*( ), Ruixiang Li*(
), Ruixiang Li*( )
)
Received:2025-03-14
Revised:2025-04-14
Published:2025-04-24
Contact:
*E-mail:liruixiang@scu.edu.cn;scuhchen@scu.edu.cn
Supported by:文章分享
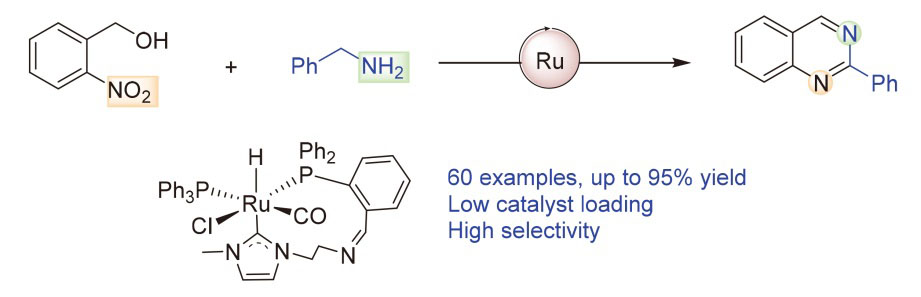
报道了以N-杂环卡宾氮膦配体(CNP)螯合的钌(II)配合物为催化剂, 以邻硝基苄醇和苄胺为底物, 通过转移氢化/环化策略高效合成喹唑啉类化合物的新方法. 催化剂的CNP配体中卡宾碳的强σ给电子能力有效稳定了催化活性物种, 氮原子的半稳定性能够为反应底物分子活化提供配位空位, 两种功能的协同作用显著提升了催化效率. 在低催化剂用量的条件下, 该体系表现出优异的催化活性和选择性, 成功实现了60种不同取代喹唑啉的合成, 最高收率可达95%, 显示了广泛的底物适用性. 通过控制实验确认了苯甲醛和苯甲亚胺为该反应的关键中间体, 并证实苄胺在该反应中同时充当了氢源和氮源的双重角色.
赵玲, 朱小慧, 陈华, 郑学丽, 薛卫超, 徐嘉麒, 付海燕, 李瑞祥. 钌催化邻硝基苯甲醇与苄胺的转移氢化/环化反应合成喹唑啉[J]. 有机化学, 2025, 45(8): 2836-2847.
Ling Zhao, Xiaohui Zhu, Hua Chen, Xueli Zheng, Weichao Xue, Jiaqi Xu, Haiyan Fu, Ruixiang Li. Synthesis of Quinazoline through Ruthenium-Catalyzed Hydrogen Transfer/Annulation Reaction between 2-Nitrobenzyl Alcohol and Benzylamine[J]. Chinese Journal of Organic Chemistry, 2025, 45(8): 2836-2847.
| Entry | Deviation from the standard conditions | Yield/% |
|---|---|---|
| 1 | None | 77 (65)c |
| 2 | No base | 38 |
| 3 | K3PO4 instead of K2HPO4 | 47 |
| 4 | Na2HPO4 instead of K2HPO4 | 51 |
| 5 | DMF instead of DMSO | 67 |
| 6 | o-Xylene instead of DMSO | 50 |
| 7 | PhCl instead of DMSO | 48 |
| 8 | 120 ℃ | 54 |
| 9 | κ3-CNP-Ru instead of κ2-CP-Ru | 75 |
| 10 | [RuH(CO)(PPh3)3Cl instead of κ2-CP-Ru | 49 |
| Entry | Deviation from the standard conditions | Yield/% |
|---|---|---|
| 1 | None | 77 (65)c |
| 2 | No base | 38 |
| 3 | K3PO4 instead of K2HPO4 | 47 |
| 4 | Na2HPO4 instead of K2HPO4 | 51 |
| 5 | DMF instead of DMSO | 67 |
| 6 | o-Xylene instead of DMSO | 50 |
| 7 | PhCl instead of DMSO | 48 |
| 8 | 120 ℃ | 54 |
| 9 | κ3-CNP-Ru instead of κ2-CP-Ru | 75 |
| 10 | [RuH(CO)(PPh3)3Cl instead of κ2-CP-Ru | 49 |
| [1] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
| [2] |
(a)
doi: 10.1021/acs.joc.8b00327 pmid: 29649359 |
|
(b)
pmid: 29649359 |
|
|
(c)
pmid: 29649359 |
|
|
(d)
pmid: 29649359 |
|
|
(e)
pmid: 29649359 |
|
| [3] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
| [4] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
| [5] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
| [6] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
| [7] |
(a)
|
|
(刘子琳, 张小洁, 张恒, 姜辉, 赵雪梅, 石林林, 朱新举, 郝新奇, 宋毛平, 有机化学, 2020, 42, 2755.)
|
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
| [8] |
(a)
|
|
(b)
doi: 10.1039/c8gc03744b |
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
| [9] |
doi: 10.1039/c7ob01159h pmid: 28660261 |
| [10] |
|
| [11] |
(a)
|
|
(b)
|
|
| [12] |
|
| [13] |
(a)
|
|
(b)
|
|
| [14] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
| [15] |
(a)
|
|
(b)
|
|
| [16] |
|
|
(王梅, 龚慧华, 付海燕, 郑学丽, 陈华, 李瑞祥, 有机化学, 2022, 42, 2418.)
doi: 10.6023/cjoc202204039 |
|
| [17] |
|
| [1] | 马豪杰, 周风院, 何雨蒙, 周小强, 孙洲, 张玉琦. 碘促进分子间环化合成2-苯基喹唑啉[J]. 有机化学, 2025, 45(6): 2157-2162. |
| [2] | 曹香雪, 贾雅会, 殷世纪, 徐亮, 韦玉, 宋欢欢. 可见光诱导二氢喹唑啉酮碳碳键断裂与三氟甲基取代烯烃的脱氟烷基化反应研究[J]. 有机化学, 2024, 44(5): 1549-1557. |
| [3] | 张文生, 郑伟, 左国强, 马科友, 肖合全, 刘改云. 一锅法还原胺化/N-酰基化/Aza-Wittig反应合成3,4-二氢喹唑啉[J]. 有机化学, 2024, 44(5): 1686-1690. |
| [4] | 吴际伟, 何俊, 王晶晶, 李丽霞, 徐采玉, 周洁, 李子荣, 许华建. 电化学氧化α-酮酸与邻氨基苄胺的脱羧环化反应[J]. 有机化学, 2024, 44(3): 972-980. |
| [5] | 周然, 袁春梅, 张桃, 毛飘, 刘燚, 孟开妮, 幸惠, 薛伟. 含喹唑啉酮的查尔酮衍生物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(9): 3196-3209. |
| [6] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [7] | 汪蕾, 于淑晶, 杨娜, 王宝雷. 新型含二氢喹唑啉酮的咖啡因衍生物的合成及生物活性研究[J]. 有机化学, 2023, 43(1): 299-307. |
| [8] | 刘威琴, 邵利辉, 李成朋, 邹雅玉, 龙海洮, 李焱, 戈强胜, 王贞超, 欧阳贵平. 3-腙喹唑啉酮衍生物的合成及抗肿瘤活性研究[J]. 有机化学, 2023, 43(1): 214-222. |
| [9] | 梁光平, 王维, 朱绪秀, 梁光焰, 杨俊, 王道平. 新型齐多夫定与4-苯胺喹唑啉骨架拼接产物的合成及体外抗肿瘤活性[J]. 有机化学, 2022, 42(9): 2793-2805. |
| [10] | 潘振涛, 刘彤, 马永敏, 颜剑波, 王亚军. 布朗斯特酸/可见光氧化还原接力催化构建喹唑啉(硫)酮[J]. 有机化学, 2022, 42(9): 2823-2831. |
| [11] | 喻晓哓, 柏汪恒, 朱建业, 张雨婷, 张梦茹, 吴际伟. 碘化铵催化sp3 C—H双胺化反应合成喹唑啉-4(3H)-酮[J]. 有机化学, 2022, 42(8): 2449-2455. |
| [12] | 王梅, 龚慧华, 付海燕, 郑学丽, 陈华, 李瑞祥. 金属钌配合物催化醇与腈串联反应合成α-取代酰胺[J]. 有机化学, 2022, 42(8): 2418-2427. |
| [13] | 陈伟, 雷思敏, 兰雨欣, 许豪键, 余坪槟, 张锐, 吴润, 陈阳. 新型喹唑啉酮衍生物的设计合成与抗植物病原真菌活性研究[J]. 有机化学, 2022, 42(7): 2164-2171. |
| [14] | 李玉东, 李莹, 董亚楠, 夏春谷, 李跃辉. 锰催化的碳酸乙烯亚乙酯对喹唑啉酮的C—H烯丙基化[J]. 有机化学, 2022, 42(3): 847-853. |
| [15] | 张瑞芹, 马仁超, 傅琴姣, 陈静, 马永敏. I2/PhNO2介导的芳乙酮C(CO)—C键氧化断裂和2-氨基芳甲酰胺胺化合成喹唑啉-4(3H)-酮[J]. 有机化学, 2022, 42(3): 854-862. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||