有机化学 ›› 2020, Vol. 40 ›› Issue (9): 2742-2754.DOI: 10.6023/cjoc202003056 上一篇 下一篇
综述与进展
刘颖杰, 孟建萍, 李晨, 林立青, 许颖
收稿日期:2020-03-24
修回日期:2020-05-03
发布日期:2020-05-29
通讯作者:
刘颖杰, 许颖
E-mail:liuyj691@nenu.edu.cn;894132290@qq.com
基金资助:Liu Yingjie, Meng Jianping, Li Chen, Lin Liqing, Xu Ying
Received:2020-03-24
Revised:2020-05-03
Published:2020-05-29
Supported by:文章分享
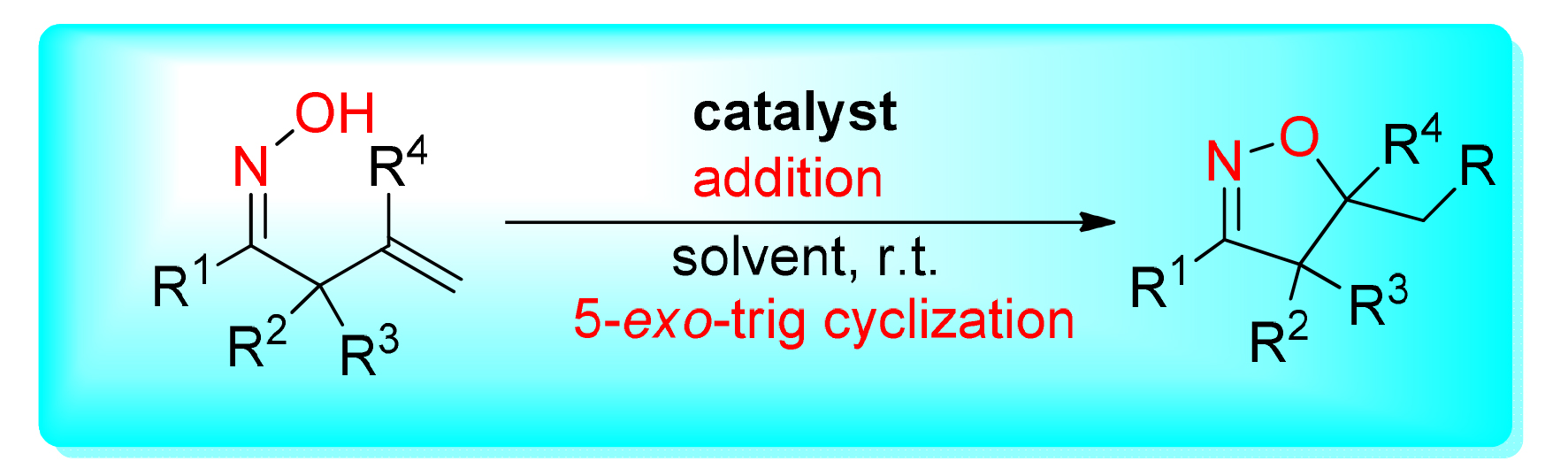
异噁唑啉是一类具有诸多生物学特性的杂环,是许多天然产物和生物活性化合物的关键结构骨架,有效的异噁唑啉合成方法已成为广泛的研究主题.描述了利用烯丙基肟合成各种官能化异噁唑啉的最新研究进展,其涉及在氧化剂存在下的自由基氧化/环化反应.这些反应通常使用容易获得的氧化剂和不同的金属或无金属作为催化剂,在中性反应条件下进行反应.
刘颖杰, 孟建萍, 李晨, 林立青, 许颖. 烯丙基肟的环化反应合成异噁唑啉衍生物的研究进展[J]. 有机化学, 2020, 40(9): 2742-2754.
Liu Yingjie, Meng Jianping, Li Chen, Lin Liqing, Xu Ying. Progress in the Synthesis of Isoxazoline Derivatives by Cycloylation of Allyl Oxime[J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2742-2754.
| [1] (a) Narasaka, K.; Kitamura, M. Eur. J. Org. Chem. 2005, 21, 4505. (b) Kassa, J.; Kuca, K.; Bartosova, L.; Kunesova, G. Curr. Org. Chem. 2007, 11, 267. [2] For recent selected examples, see:(a) Sato, Y.; Kawaguchi, S.; Nomoto, A.; Ogawa, A. Angew. Chem.. Int. Ed. 2016, 55, 9700. (b) Zhao, J.; Li, P.; Li, X.; Xia, C.; Li, F. Chem. Commun. 2016, 52, 3661. (c) Cui, H.; Liu, X.; Wei, W.; Yang, D.; He, C.; Zhang, T.; Wang, H. J. Org. Chem. 2016, 81, 2252. (d) Lan, X.-W.; Wang, N.-X.; Bai, C.-B.; Lan, C.-L.; Zhang, T.; Chen, S.-L.; Xing, Y. Org. Lett. 2016, 18, 5986. [3] Pozharskii, A. F.; Katritzky, A. R.; Soldatenkov, A. Heterocycles in Life and Society:An Introduction to Heterocyclic Chemistry. Biochemistry and Applications, John Wiley Sons, New Jersey, 2011, pp. 35~62. [4] Xu, Z.; Ye, T. Heterocycles in Natural Product Synthesis, Wiley-VCH, Darmstadt, 2011, pp. 459~505. [5] Alvarez-Builla, J.; Vaquero, J. J.; Barluenga, J. Modern Heterocyclic Chemistry, Wiley-VCH, Darmstadt, 2011, pp. 989~1045. [6] Li, J. J.; Hruby, V. Angew. Chem.. Int. Ed. 2017, 129, 2583. [7] Selected examples of biological evaluation of isoxazolines:(a) Castellano, S.; Kuck, D.; Viviano, M.; Yoo, J.; López-Vallejo, F.; Conti, P.; Tamborini, L.; Pinto, A.; Medina-Franco, J. L.; Sbardella, G. J. J. Med. Chem. 2011, 54, 7663. (b) Poutiainen, P. K.; Venäläinen, T. A.; Peräkylä, M.; Matilainen, J. M.; Väisänen, S.; Honkakoski, P.; Laatikainen, R.; Pulkkinen, J. T. Bioorg. Med. Chem. 2010, 18, 3437. (c) Namboothiri, I. N. N.; Rastogi, N. Isoxazolines from Nitro Compounds. Synthesis and Applications, Springer, Berlin, 2008, pp. 1~44. (d) Bode, J. W.; Carreira, E. M. Org. Lett. 2001, 3, 1587. [8] For selected reviews, see:(a) Rück-Braun, K.; Freysoldt, T. H. E.; Wierschem, F. Chem. Soc. Rev. 2005, 34, 507. (b) Kaur, K.; Kumar, V.; Sharma, A. K.; Gupta, G. K. Eur. J. Med. Chem. 2014, 77, 121. (c) Hu, F.; Szostak, M. Adv. Synth. Catal. 2015, 357, 2583. (d) Morita, T.; Yugandar, S.; Fuse, S.; Nakamura, H. Tetrahedron Lett. 2018, 59, 1159. [9] Zaki, Y. H.; Sayed, A. R.; Elroby, S. A. Chem. Cent. J. 2016, 10, 17. [10] Suresh, G.; Nadh, R. V.; Srinivasu, N.; Kaushal, K. Synth. Commun. 2016, 46, 1972. [11] Ning, G. H.; Zhao, W. T.; Bian, Q.; Tang, X. Y. Chin. J. Org. Chem. 2014, 34, 1800(in Chinese). (宁国慧, 赵温涛, 边强, 唐向阳, 有机化学, 2014, 34, 1800.) [12] Jiang, S.; Tsikolia, M.; Bernier, U. R.; Bloomquist, J. R. Int. J. Environ. Res. Public Health 2017, 14, 154. [13] Filali, I.; Bouajila, J.; Znati, M.; Garah, B.-E.; Jannet, H. B. J. Med. Chem. 2015, 30, 371. [14] Khazir, J.; Singh, P. P.; Reddy, D. M.; Hyder, I.; Shafi, S.; Sawant, S. D.; Chashoo, G.; Mahajan, A.; Alamc, M. S.; Saxena, A. K.; Arvinda, S.; Gupta, B. D.; Kumara, H. M. S. Eur. J. Med. Chem. 2013, 63, 279. [15] Xue, C.; Wityak, J.; Sielecki, T. M.; Pinto, D. J.; Batt, D. G.; Cain, G. A.; Sworin, M.; Rockwell, A. L.; Roderick, J. J.; Wang, S.; Orwat, M. J.; Frietze, W. E.; Bostrom, L. L.; Liu, J.; Higley, C. A.; Rankin, F. W.; Tobin, A. E.; Emmett, G.; Lalka, G. K.; Sze, J. Y.; DiMeo, S. V.; Mousa, S. A.; Thoolen, M. J.; Racanelli, A. L.; Hausner, E. A.; Reilly, T. M.; DeGrado, W. F.; Wexler, R. R.; Olson, R. E. J. Med. Chem. 1997, 40, 2064. [16] Cheng, K. F.; Al-Abed, Y. Bioorg. Med. Chem. Lett. 2006, 16, 3376. [17] Goyard, D.; Kónya, B.; Chajistamatiou, A. S.; Chrysina, E. D.; Leroy, J.; Balzarin, S.; Tournier, M.; Tousch, D.; Petit, P.; Duret, C.; Maurel, P.; Somsák, L.; Docsa, T.; Gergely, P.; Praly, J.; Azay-Milhau, J.; Vidal, S. Eur. J. Med. Chem. 2016, 108, 444. [18] (a) Talley, J. J.; Brown, D. L.; Carter, J. S.; Graneto, M. J.; Koboldt, C. M.; Masferrer, J. L.; Perkins, W. E.; Rogers, R. S.; Shaffer, A. F.; Zhang, Y. Y.; Zweifel, B. S.; Seibert, K. J. Med. Chem. 2000, 43, 775. (b) Talley, J. J.; Bertenshaw, S. R.; Brown, D. L.; Carter, J. S.; Graneto, M. J.; Kellogg, M. S.; Koboldt, C. M.; Yuan, J.; Zhang, Y. Y.; Seibert, K. J. Med. Chem. 2000, 43, 1661. [19] Taylor, R. D.; MacCoss, M.; Lawson, A. D. G. J. Med. Chem. 2014, 57, 5845. [20] (a) Minter, A. R.; Fuller, A. A.; Mapp, A. K. J. Am. Chem. Soc. 2003, 125, 6846. (b) Fuller, A. A.; Chen, B.; Minter, A. R.; Mapp, A. K. J. Am. Chem. Soc. 2005, 127, 5376. [21] Jiang, D.; Peng, J.; Chen, Y. Org. Lett. 2008, 10, 1695. [22] Marotta, E.; Micheloni, E.; Scardovi, N.; Righi, P. Org. Lett. 2001, 3, 727. [23] For selected reviews, see:(a) Kiss, L.; Nonn, M.; Fülöp, F. Synthesis 2012, 44, 1951. (b) Vitale, P.; Scilimati, A. Synthesis 2013, 45, 2940. [24] Yoshimura, A.; Middleton, K. R.; Todora, A. D.; Kastern, B. J.; Koski, S. R.; Maskaev, A. V.; Zhdankin, V. V. Org. Lett. 2013, 15, 4010. [25] For selected examples on the cycloaddition of oximes withalkenes, see:(a) Zhu, M.-K.; Zhao, J.-F.; Loh, T.-P. J. Am. Chem. Soc. 2010, 132, 6284. (b) Han, B.; Yang, X.-L.; Fang, R.; Yu, W.; Wang, C.; Duan, X.-Y.; Liu, S. Angew. Chem.. Int. Ed. 2012, 51, 8816. (c) Schmidt, E. Y.; Tatarinova, I. V.; Ivanova, E. V.; Zorina, N. V.; Ushakov, I. A.; Trofimov, B. A. Org. Lett. 2013, 15, 104. (d) Dong, J.; Ding, T.; Zhang, S.; Chen, Z.; Tu, Y. Angew. Chem.. Int. Ed. 2018, 57, 13192. [26] For selected examples on the cycloaddition of nitro com-pounds with alkenes, see:(a) Cecchi, L.; DeSarlo, F.; Machetti, F. Chem.-Eur. J. 2008, 14, 7903. (b) Trogu, E.; DeSarlo, F.; Machetti, F. Chem.-Eur. J. 2009, 15, 7940. (c) Chary, R. G.; Reddy, G. R.; Ganesh, Y. S. S.; Prasad, K. V.; Raghunadh, A.; Krishna, T.; Mukherjee, S.; Pal, M. Adv. Synth. Catal. 2014, 356, 160. [27] Han, B.; Yang, X.-L.; Fang, R.; Yu, W.; Wang, C.; Duan, X.-Y.; Liu, S. Angew. Chem.. Int. Ed. 2012, 51, 8816. [28] For reviews, see:(a) Zard, S. Z. Chem. Soc. Rev. 2008, 37, 1603. (b) Hartung, J. Eur. J. Org. Chem. 2001, 4, 619. (c) Jasperse, C. P.; Curran, D. P.; Fevig, T. L. Chem. Rev. 1991, 91, 1237. (d) Song, L.; Liu, K.; Li, C. Org. Lett. 2011, 13, 3434. (e) Liu, F.; Liu, K.; Yuan, X.; Li, C. J. Org. Chem. 2007, 72, 10231. [29] Jiang, D. H.; Peng, J. S.; Chen, Y. W. Org. Lett. 2008, 10, 1695. [30] Zhu, M.-K.; Zhao, J.-F.; Loh, T.-P. J. Am. Chem. Soc. 2010, 132, 6284. [31] Dong, K.-Y.; Qin, H.-T.; Bao, X.-X.; Liu, F.; Zhu, C. Org. Lett. 2014, 16, 5266. [32] For selected reviews on oxidation reactions with molecular oxygen, see:(a) Mukaiyama, T.; Yamada, T. Bull. Chem. Soc. Jpn. 1995, 68, 17. (b) Punniyamurthy, T.; Velusamy, S.; Iqbal, J. Chem. Rev. 2005, 105, 2329. (c) Piera, J.; Bäckvall, J.-E. Angew. Chem.. Int. Ed. 2008, 47, 3506. (d) Wendlandt, A. E.; Suess, A. M.; Stahl, S. S. Angew. Chem.. Int. Ed. 2011, 50, 11062. (e) Shi, Z.; Zhang, C.; Tang, C.; Jiao, N. Chem. Soc. Rev. 2012, 41, 3381. [33] (a) Bag, R.; Sar, D.; Punniyamurthy, T. Org. Lett. 2015, 17, 2010. (b) Andia, A. A.; Miner, M. R.; Woerpel, K. A. Org. Lett. 2015, 17, 2704. (c) Lu, Q.; Liu, Z.; Luo, Y.; Zhang, G.; Huang, Z.; Wang, H.; Liu, C.; Miller, J. T.; Lei, A. Org. Lett. 2015, 17, 3402. (d) Lu, Q.; Peng, P.; Luo, Y.; Zhao, Y.; Zhou, M.; Lei, A. Chem.-Eur. J. 2015, 21, 18580. (e) Xia, X.-F.; Zhu, S.-L.; Gu, Z.; Wang, H.; Li, W.; Liu, X.; Liang, Y.-M. J. Org. Chem. 2015, 80, 5572. (f) Zhang, J.-Z.; Tang, Y. Adv. Synth. Catal. 2016, 358, 752. (g) Miner, M. R.; Woerpel, K. A. Eur. J. Org. Chem. 2016, 34, 1860. (h) Bag, R.; Sar, D.; Punniyamurthy, T. Org. Biomol. Chem. 2016, 14, 3246. [34] For the metal-free aerobic dioxygenation of C=C bonds using hydrox-amic acids, see:(a) Schmidt, V. A.; Alexanian, E. J. Angew. Chem.. Int. Ed. 2010, 49, 4491. (b) Giglio, B. C.; Schmidt, V. A.; Alexanian, E. J. J. Am. Chem. Soc. 2011, 133, 13320. (c) Schmidt, V. A.; Alexanian, E. J. Chem. Sci. 2012, 3, 1672. [35] For the aerobic oxytrifluoromethylation of C=C bonds, see:(a) Zhang, C.-P.; Wang, Z.-L.; Chen, Q.-Y.; Zhang, C.-T.; Gu, Y.-C.; Xiao, J.-C. Chem. Commun. 2011, 47, 6632. (b) Deb, A.; Manna, S.; Modak, A.; Patra, T.; Maity, S.; Maiti, D. Angew. Chem.. Int. Ed. 2013, 52, 9747. (c) Yang, Y.; Liu, Y.; Jiang, Y.; Zhang, Y.; Vicic, D. J. Org. Chem. 2015, 80, 6639. (d) Liu, C.; Lu, Q.; Huang, Z.; Zhang, J.; Liao, F.; Peng, P.; Lei, A. Org. Lett. 2015, 17, 6034. [36] For the aerobic oxy-2,2,2-trifluoroethylation of C=C bonds, see:Li, L.; Huang, M.; Liu, C.; Xiao, J.-C.; Chen, Q.-Y.; Guo, Y.; Zhao, Z.-G. Org. Lett. 2015, 17, 4714. [37] For the aerobic oxysulfonylation of C=C bonds, see:(a) Lu, Q.; Zhang, J.; Wei, F.; Qi, Y.; Wang, H.; Liu, Z.; Lei, A. Angew. Chem.. Int. Ed. 2013, 52, 7156. (b) Wei, W.; Liu, C.; Yang, D.; Wen, J.; You, J.; Suo, Y.; Wang, H. Chem. Commun. 2013, 49, 10239. [38] Keshari, T.; Ya-dav, V. K.; Srivastava, V. P.; Yadav, L. D. S. Green Chem. 2014, 16, 3986. [39] Zhou, S.-F.; Pan, X.; Zhou, Z.-H.; Shoberu, A.; Zou, J.-P. J. Org. Chem. 2015, 80, 3682. [40] Wei, W.; Ji, J.-X. Angew. Chem.. Int. Ed. 2011, 50, 9097. [41] Sun, X.; Li, X.; Song, S.; Zhu, Y.; Liang, Y.-F.; Jiao, N. J. Am. Chem. Soc. 2015, 137, 6059. [42] For autoxidative 4,5-dihydroisoxazoline ring formation through thenitrooxylation of C=C bonds using stoichiometric amounts of tert-butylnitrite, see:Zhang, X.-W.; Xiao, Z.-F.; Zhuang, Y.-J.; Wang, M.-M.; Kang, Y.-B. Adv. Synth. Catal. 2016, 358, 1942. [43] (a) Castellano, S.; Kuck, D.; Vivi-ano, M.; Yoo, J.; López-Vallejo, F.; Conti, P.; Tamborini, L.; Pinto, A.; Medina-Franco, J. L.; Sbardella, G. J. Med. Chem. 2011, 54, 7663. (b) Patel, N. C.; Schwarz, J.; Hou, X. J.; Hoover, D. J.; Xie, L.; Fliri, A. J.; Gallaschun, R. J.; Lazzaro, J. T.; Bryce, D. K.; Hoffmann, W. E.; Hanks, A. N.; McGinnis, D.; Marr, E. S.; Gazard, J. L.; Hajós, M.; Scialis, R. J.; Hurst, R. S.; Shaffer, C. L.; Pandit, J.; O'Donnell, C. J. J. Med. Chem. 2013, 56, 9180. (c) Rodrigues, G. C.; Feijó, D. F.; Bozza, M. T.; Pan, P.; Vullo, D.; Parkkila, S.; Supuran, C. T.; Capasso, C.; Aguiar, A. P.; Vermelho, A. B. J. Med. Chem. 2014, 57, 298. [44] (a) Nishikawa, N. Method and Applications of Cycloaddition Reactions in Organic Syntheses, Wiley, Hoboken, NJ, 2014, pp. 565~598. (b) Feuer, H. Nitrile Oxides. Nitrones. and Nitronates in Organic Synthesis. Novel Strategies in Synthesis, Wiley, Hoboken, NJ, 2008, p. 760. (c) Kotyatkina, A. I.; Zhabinsky, V. N.; Khripach, V. A. Russ. Chem. Rev. 2001, 70, 641. (d) Kozikowski, A. P. Acc. Chem. Res. 1984, 17, 410. [45] Yamamoto, D.; Oguro, T.; Tashiro, Y.; Soga, M.; Miyashita, K.; Aso, Y.; Makino, K. Eur. J. Org. Chem. 2016, 31, 5216. [46] Yamamoto, D.; Soga, M.; Ansai, H.; Makino, K. Org. Chem. Front. 2016, 3, 1420. [47] (a) Shcroft, C. P.; Hellier, P.; Pettman, A.; Wakinson, S. Org. Process Res. Dev. 2011, 15, 98. (b) Ettari, R.; Nizi, E.; Franceco, M. E. D.; Dude, M.-A.; Pradel, G.; Vičík, R.; Schirmeister, T.; Micale, N.; Grasso, S.; Zappalà, M. J. Med. Chem. 2008, 51, 988. (c) Reck, F.; Zhou, F.; Girardot, M.; Kern, G.; Eyermann, C. J.; Hales, N. J.; Ramsay, R. R.; Gravestock, M. B. J. Med. Chem. 2005, 48, 499. [48] (a) Sun, X.; Yu, F.; Ye, T.; Liang, X.; Ye, J. Chem.-Eur. J. 2011, 17, 430. (b) El-Awa, A.; Noshi, M. N.; du Jourdin, X. M.; Fuchs, P. L. Chem. Rev. 2009, 109, 2315. (c) Plesniak, K.; Zarecki, A.; Wicha, J. Top. Curr. Chem. 2007, 275, 163. [49] Gao, Y.; Tang, X.; Peng, J.; Hu, M.; Wu, W.; Jiang, H. Org. Lett. 2016, 18, 1158. [50] Wang, L.-J.; Chen, M.-M.; Qi, L.; Xu, Z.-D.; Li, W. Chem. Commun. 2017, 52, 2056. [51] (a) Li, Y.; Studer, A. Angew. Chem.. Int. Ed. 2012, 51, 8221. (b) Zhang, B.; Studer, A. Org. Lett. 2013, 15, 4548. [52] Lv, Y. H.; Pu, W. Y.; Wang, Q. Q.; Chen, Q.; Niu, J. J.; Zhang, Q. Adv. Synth. Catal. 2017, 359, 3114. [53] Sun, K.; Li, G. F.; Li, Y. Y.; Yu, J.; Zhao, Q.; Zhang, Z. G.; Zhang, G. S. Adv. Synth. Catal. 2020, 362, 10. [54] Wang, X.; Li, G. F.; Sun, K.; Zhang, B. Chin. J. Org. Chem. 2020, 40, 913(in Chinese). (王薪, 李国锋, 孙凯, 张冰, 有机化学, 2020, 40, 913.) [55] Sun, K.; Wang, S. N.; Feng, R. R.; Zhang, Y. X.; Wang, X.; Zhang, Z. G.; Zhang, B. Org. Lett. 2019, 21, 2052. [56] Liu, X.-Y.; Li, X.-T.; Gu, Q.-S.; Dong, X.-Y.; Meng, X. Angew. Chem.. Int. Ed. 2018, 57, 7668. [57] (a) Zhu, R.; Buchwald, S. L. Angew. Chem.. Int. Ed. 2013, 52, 12655. (b) Zhu, R.; Buchwald, S. L. J. Am. Chem. Soc. 2015, 137, 8069. (c) Zhang, W.; Wang, F.; McCann, S. D.; Wang, D.; Chen, P.; Stahl, S. S.; Liu, G. Science 2016, 353, 1014. (d) Wang, F.; Wang, D.; Wan, X.; Wu, L.; Chen, P.; Liu, G. J. Am. Chem. Soc. 2016, 138, 15547. (e) Wang, D.; Wang, F.; Chen, P.; Lin, Z.; Liu, G. Angew. Chem.. Int. Ed. 2017, 56, 2054. (f) Wu, L.; Wang, F.; Wan, X.; Wang, D.; Chen, P.; Liu, G. J. Am. Chem. Soc. 2017, 139, 2904. [58] (a) Lin, J.-S.; Dong, X.-Y.; Li, T.-T.; Jiang, N.-C.; Tan, B.; Liu, X.-Y. J. Am. Chem. Soc. 2016, 138, 9357. (b) Lin, J.-S.; Wang, F.-L.; Dong, X.-Y.; He, W.-W.; Yuan, Y.; Chen, S.; Liu, X.-Y. Nat. Commun. 2017, 8, 14841. (c) Cheng, Y.-F.; Dong, X.-Y.; Gu, Q.-S.; Yu, Z.-L.; Liu, X.-Y. Angew. Chem.. Int. Ed. 2017, 56, 8883. (d) Wang, F.-L.; Dong, X.-Y.; Lin, J.-S.; Zeng, Y.; Jiao, G.-Y.; Gu, Q.-S.; Guo, X.-Q.; Ma, C.-L.; Liu, X.-Y. Chem. Commun. 2017, 3, 979. [59] For selected references on Cu(II)-O(N) species and reactions involvingalkyl radical intermediates, see:(a) Huffman, L. M.; Stahl, S. S. J. Am. Chem. Soc. 2008, 130, 9196. (b) Creutz, S. E.; Lotito, K. J.; Fu, G. C.; Peters, J. C. Science 2012, 338, 647. (c) Tran, B. L.; Li, B.; Driess, M.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 2555. [60] For selected examples, see:(a) He, Y.-T.; Li, L.-H.; Yang, Y.-F.; Wang, Y.-Q.; Luo, J.-Y.; Liu, X.-Y.; Liang, Y.-M. Chem. Commun. 2013, 49, 5687. (b) Wei, Q.; Chen, J.-R.; Hu, X.-Q.; Yang, X.-C.; Lu, B.; Xiao, W.-J. Org. Lett. 2015, 17, 4464. (c) Zhang, W.; Su, Y.; Wang, K.-H.; Wu, L.; Chang, B.; Shi, Y.; Huang, D.; Hu, Y. Org. Lett. 2017, 19, 376. [61] Mukaiyama, T.; Yamada, T. Bull. Chem. Soc. Jpn. 1995, 68, 17. [62] Li, W.; Jia, P.; Han, B.; Li, D.; Yu, W. Tetrahedron 2013, 69, 3274. [63] Peng, X.-X.; Deng, Y.-J.; Yang, X.-L.; Zhang, L.; Yu, W.; Han, B. Org. Lett. 2014, 16, 4650. [64] (a) Chen, Y.-X.; Qian, L.-F.; Zhang, W.; Han, B. Angew. Chem. 2008, 120, 9470. (b) Han, B.; Wang, C.; Han, R.-F.; Yu, W.; Duan, X.-Y.; Fang, R.; Yang, X.-L. Chem. Commun. 2011, 47, 7818. (c) Han, B.; Yang, X.-L.; Wang, C.; Bai, Y.-W.; Pan, T.-C.; Chen, X.; Yu, W. J. Org. Chem. 2012, 77, 1136. (d) Han, B.; Han, R.-F.; Ren, Y.-W.; Duan, X.-Y.; Xu, Y.-C.; Zhang, W. Tetrahedron 2011, 67, 5615. [65] (a) Pratt, D. A.; Blake, J. A.; Mulder, P.; Walton, J. C.; Korth, H.-G.; Ingold, K. U. J. Am. Chem. Soc. 2004, 126, 10667. (b) Chong, S.-S.; Fu, Y.; Liu, L.; Guo, Q.-X. J. Phys. Chem. A 2007, 111, 13112. [66] For transition metal catalyzed vicinal dioxygenation, oxyamination, and diamination of olefins, see:(a) Kolb, H. C.; VanNieuwenhze, M. S.; Sharpless, K. B. Chem. Rev. 1994, 94, 2483. (b) Wang, A.; Jiang, H.; Chen, H. J. Am. Chem. Soc. 2009, 131, 3846. (c) Zhang, Y.; Sigman, M. S. J. Am. Chem. Soc. 2007, 129, 3076. (d) Fuller, P. H.; Kim, J.-W.; Chemler, S. R. J. Am. Chem. Soc. 2008, 130, 17638. (e) Williamson, K. S.; Yoon, T. P. J. Am. Chem. Soc. 2010, 132, 4570. (f) Du, H.; Zhao, B.; Shi, Y. J. Am. Chem. Soc. 2008, 130, 8590. [67] Selected examples of biological evaluation of isoxazolines:(a) Castellano, S.; Kuck, D.; Viviano, M.; Yoo, J.; López-Vallejo, F.; Conti, P.; Tamborini, L.; Pinto, A.; Medina-Franco, J. L.; Sbardella, G. J. J. Med. Chem. 2011, 54, 7663. (b) Poutiainen, P. K.; Venäläinen, T. A.; Peräkylä, M.; Matilainen, J. M.; Väisänen, S.; Honkakoski, P.; Laatikainen, R.; Pulkkinen, J. T. Bioorg. Med. Chem. 2010, 18, 3437. (c) Bode, J. W.; Carreira, E. M. Org. Lett. 2001, 3, 1587. (d) Curran, D. P.; Heffner, T. A. J. Org. Chem. 1990, 55, 4585. (e) Kozikowski, A. P. Acc. Chem. Res. 1984, 17, 410. (f) Jiang, D.; Peng, J.; Chen, Y. Org. Lett. 2008, 10, 1695. (g) Zhu, M.-K.; Zhao, J.-F.; Loh, T.-P. J. Am. Chem. Soc. 2010, 132, 6284. (h) He, Y.-H.; Li, L.-H.; Yang, Y.-F.; Wang, Y.-Q.; Luo, J.-Y.; Liu, X.-Y.; Liang, Y.-M. Chem. Commun. 2013, 49, 5687. (i) Tripathi, C. B.; Mukherjee, S. Angew. Chem.. Int. Ed. 2013, 52, 8450. [68] Some selected reviews:(a) Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523. (b) Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299. (c) Schafer, S.; Wirth, T. Angew. Chem.. Int. Ed. 2010, 49, 2786. (d) Dohi, T.; Kita, Y. Chem. Commun. 2009, 16, 2073. (e) Ngatimin, M.; Lupton, D. W. Aust. J. Chem. 2010, 63, 653. (f) Uyanik, M.; Ishihara, K. Chem. Commun. 2009, 16, 2086. (g) Ciufolini, M. A.; Braun, N. A.; Canesi, S.; Ousmer, M.; Chang, J.; Chai, D. Synthesis 2007, 3759. [69] Some selected examples where polyvalent iodine reagents mediate alkene difunctionalization:(a) Lovick, H. M.; Michael, F. E. J. Am. Chem. Soc. 2010, 132, 1249. (b) Wardrop, D. J.; Bowen, E. G.; Forslund, R. E.; Sussman, A. D.; Weerasekera, S. L. J. Am. Chem. Soc. 2010, 132, 1188. (c) Kang, Y.-B.; Gade, L. H. J. Am. Chem. Soc. 2011, 133, 3658. (d) Röben, C.; Souto, J. A.; Gonzálz, Y.; Lishchynskyi, A.; Muńiz, K. Angew. Chem.. Int. Ed. 2011, 50, 9478. (e) Cochran, B. M.; Michael, F. E. Org. Lett. 2008, 10, 5039. (f) Correa, A.; Tellitu, I.; Domínguez, E.; Sanmartin, R. J. Org. Chem. 2006, 71, 8316. (g) Muńiz, K.; Hövelmann, C. H.; Campos-Gómez, E.; Barluenga, J.; González, J. M.; Streuff, J.; Nieger, M. Chem.-Asian J. 2008, 3, 776. (h) Li, H.; Widenhoefer. Tetrahedron 2010, 66, 4827. (i) Fujita, M.; Wakita, W.; Sugimura, T. Chem. Commun. 2011, 47, 3983. [70] (a) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881. (b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320. [71] Kong, W.; Guo, Q.; Xu, Z.; Wang, G.; Jiang, X.; Wang, R. Org. Lett. 2015, 17, 3686. [72] Yu, J.-M.; Cai, C. Org. Biomol. Chem. 2018, 16, 490. [73] (a) Chen, F.; Yang, X. L.; Wu, Z. W.; Han, B. J. Org. Chem. 2016, 81, 3042. (b) Duan, X. Y.; Yang, X. L.; Fang, R.; Peng, X. X.; Yu, W.; Han, B. J. Org. Chem. 2013, 78, 10692. [74] Peng, X. X.; Deng, Y. J.; Yang, X. L.; Zhang, L.; Yu, W.; Han, B. Org. Lett. 2014, 16, 4650. [75] Yang, X. L.; Chen, F.; Zhou, N. N.; Yu, W.; Han, B. Org. Lett. 2014, 16, 6476. [76] (a) Kong, W.; Guo, Q.; Xu, Z.; Wang, G.; Jiang, X.; Wang, R. Org. Lett. 2015, 17, 3686. (b) Hu, X. Q.; Feng, G.; Chen, J. R.; Yan, D. M.; Zhao, Q. Q.; Wei, Q.; Xiao, W. J. Org. Biomol. Chem. 2015, 13, 3457. [77] Zhang, X.-W.; Xiao, Z.-F.; Zhuang, Y.-J.; Wang, M.-M.; Kang, Y.-B. Adv. Synth. Catal. 2016, 358, 1942. [78] For iminoxyl radicals proved by EPR spectroscopy orother methods, see:(a) Zhu, X.; Wang, Y.-F.; Ren, W.; Zhang, F.-L.; Chiba, S. Org. Lett. 2013, 15, 3214. (b) Liu, Y.-Y.; Yang, X.-H.; Yang, J.; Song, R.-J.; Li, J.-H. Chem. Commun. 2014, 50, 6906. (c) Krylov, I. B.; Terentev, A. O.; Timofeev, V. P.; Shelimov, B. N.; Novikov, R. A.; Merkulova, V. M.; Nikishin, G. I. Adv. Synth. Catal. 2014, 356, 2266. (d) Yang, X.-L.; Chen, F.; Zhou, N.-N.; Yu, W.; Han, B. Org. Lett. 2014, 16, 6476. (e) Lemercier, B. C.; Pierce, J. G. Org. Lett. 2015, 17, 4542. [79] Peng, X.-X.; Deng, Y.-J.; Yang, X.-L.; Zhang, L.; Yu, W.; Han, B. Org. Lett. 2014, 16, 4650. [80] (a) Galliker, B.; Kissner, R.; Nauser, T.; Koppenol, W. H. Chem.-Eur. J. 2009, 15, 6161. (b) Taniguchi, T.; Yajima, A.; Ishibashi, H. Adv. Synth. Catal. 2011, 353, 2643. [81] Lopes, E. F.; Penteado, F.; Thurow, S.; Pinz, M.; Reis, A.; Wilhelm, E.; Luchese, C.; Barcellos, T.; Dalberto, B.; Alves, D.; Silva, M. S. D.; Lenardão, E. J. Org. Chem. 2019, 84, 12452. [82] Triandafillidi, I.; Kokotos, C. G. Org. Lett. 2017, 19, 4254. [83] (a) Limnios, D.; Kokotos, C. G. ACS Catal. 2013, 3, 2239. (b) Limnios, D.; Kokotos, C. G. Chem.-Eur. J. 2014, 20, 559. (c) Limnios, D.; Kokotos, C. G. J. Org. Chem. 2014, 79, 4270. (d) Theodorou, A.; Limnios, D.; Kokotos, C. G. Chem.-Eur. J. 2015, 21, 5238. [84] Zhang, W. G.; Su, Y. P.; Wang, K.-H.; Wu, L. L.; Chang, B. B.; Shi, Y.; Huang, D. F.; Hu, Y. L. Org. Lett. 2017, 19, 376. [85] (a) Tilstam, U.; Weinmann, H. Org. Process Res. Dev. 2002, 6, 384. (b) Mendonça, G. F.; Sindra, H. C.; de Almeida, L. S.; Esteves, P. M.; de Mattos, M. C. S. Tetrahedron Lett. 2009, 50, 473. (c) Mishra, A. K.; Nagarajaiah, H.; Moorthy, J. N. Eur. J. Org. Chem. 2015, 46, 2733. (d) Gaspa, S.; Porcheddu, A.; Luca, L. D. Adv. Synth. Catal. 2016, 358, 154. [86] (a) Luca, L. D.; Giacomelli, G.; Porcheddu, A. Org. Lett. 2001, 3, 3041. (b) Ye, J.; Wang, Y.; Chen, J.; Liang, X. Adv. Synth. Catal. 2004, 346, 691. (c) Sniady, A.; Morreale, M. S.; Wheeler, K. A.; Dembinski, R. Eur. J. Org. Chem. 2008, 39, 3449. (d) Jing, Y.; Daniliuc, C. G.; Studer, A. Org. Lett. 2014, 16, 4932. (e) Raihan, M. J.; Rajawinslin, R. R.; Kavala, V.; Kuo, C.-W.; Kuo, T.-S.; He, C.-H.; Huang, H.-N.; Yao, C.-F. J. Org. Chem. 2013, 78, 8872. [87] (a) Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. Angew. Chem.. Int. Ed. 2011, 50, 3793. (b) Litvinas, N. D.; Fier, P. S.; Hartwig, J. F. Angew. Chem.. Int. Ed. 2012, 51, 536. (c) Oishi, M.; Kondo, H.; Amii, H. Chem. Commun. 2009, 14, 1909. (d) Weng, Z.; Lee, R.; Jia, W.; Yuan, Y.; Wang, W.; Feng, X.; Huang, K.-W. Organometallics 2011, 30, 3229. (e) Shimizu, N.; Kondo, H.; Oishi, M.; Fujikawa, K.; Komoda, K.; Amii, H. Org. Synth. 2016, 93, 147. [88] Wei, Q.; Chen, J.-R.; Hu, X.-Q.; Yang, X.-C.; Lu, B.; Xiao, W.-J. Org. Lett. 2015, 17, 4464. [89] For reviews of difluoromethylation:(a) Rong, J.; Ni, C.; Hu, J. Asian J. Org. Chem. 2017, 6, 139. (b) Belhomme, M.-C.; Besset, T.; Poisson, T.; Pannecoucke, X. Chem.-Eur. J. 2015, 21, 12836. (c) Yerien, D. E.; Barata-Vallejo, S.; Postigo, A. Chem.-Eur. J. 2017, 23, 14676. [90] (a) Fujiwara, Y.; Dixon, J. A.; O'Hara, F.; Funder, E. D.; Dixon, D. D.; Rodri-guez, R. A.; Baxter, R. D.; Herle, B.; Sach, N.; Collins, M. R.; Ishihara, Y.; Baran, P. S. Nature 2012, 492, 95. (b) Fujiwara, Y.; Dixon, J. A.; Rodriguez, R. A.; Baxter, R. D.; Dixon, D. D.; Collins, M. R.; Blackmond, D. G.; Baran, P. S. J. Am. Chem. Soc. 2012, 134, 1494. [91] For selected examples, see:(a) Lin, Q.-Y.; Xu, X.-H.; Zhang, K.; Qing, F.-L. Angew. Chem.. Int. Ed. 2016, 55, 1479. (b) He, Z.; Tan, P.; Ni, C.; Hu, J. Org. Lett. 2015, 17, 1838. (c) Arai, Y.; Tomita, R.; Ando, G.; Koike, T.; Akita, M. Chem.-Eur. J. 2016, 22, 1262. (d) Zou, G.; Wang, X. Org. Biomol. Chem. 2017, 15, 8748. (e) Ran, Y.; Lin, Q.-Y.; Xu, X.-H.; Qing, F.-L. J. Org. Chem. 2016, 81, 7001. (f) Tang, X.-J.; Dolbier, W. R. Angew. Chem.. Int. Ed. 2015, 54, 4246. (g) Noto, N.; Koike, T.; Akita, M. J. Org. Chem. 2016, 81, 7064. (h) Tang, X.; Zhang, Z.; Dolbier, W. R. Chem.-Eur. J. 2015, 21, 18961. (i) Lin, Q.-Y.; Ran, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2016, 18, 2419. [92] Noto, N.; Koike, T.; Akita, M. Chem. Sci. 2017, 8, 6375. [93] Xiong, P.; Xu, H.-H.; Song, J. S.; Xu, H.-C. J. Am. Chem. Soc. 2018, 140, 2460. [94] Zhu, M.; Fun, W. J.; Guo, W. B.; Tian, Y. F.; Wang, Z. Q.; Xu, C.; Ji, B. M. Eur. J. Org. Chem. 2019, 14, 1614. [95] Xiao, W.-J.; Hu, X.-Q.; Chen, J.; Chen, J.-R.; Yan, D.-M. Chem.-Eur. J. 2016, 22, 14141. |
| [1] | 赵茜帆, 陈永正, 张世明. 碳基非金属催化剂在有机合成领域的应用及机理研究[J]. 有机化学, 2024, 44(1): 137-147. |
| [2] | 赵红琼, 于淼, 宋冬雪, 贾琦, 刘颖杰, 季宇彬, 许颖. 羧酸脱羧羟基化反应研究进展[J]. 有机化学, 2024, 44(1): 70-84. |
| [3] | 高晓阳, 翟锐锐, 陈训, 王烁今. 碳酸亚乙烯酯参与C—H键活化反应的研究进展[J]. 有机化学, 2023, 43(9): 3119-3134. |
| [4] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [5] | 陈新强, 张敬. 伯醇的脱羟甲基反应的研究进展[J]. 有机化学, 2023, 43(8): 2711-2719. |
| [6] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [7] | 户晓兢, 郭斐翔, 朱润青, 周柄棋, 张涛, 房立真. 对烷氧基酚的合成及其去芳构化后的合成应用[J]. 有机化学, 2023, 43(6): 2239-2244. |
| [8] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [9] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [10] | 徐光利, 许静, 徐海东, 崔香, 舒兴中. 过渡金属催化烯烃和炔烃合成1,3-共轭二烯化合物研究进展[J]. 有机化学, 2023, 43(6): 1899-1933. |
| [11] | 庞明杨, 常宏宏, 冯璋, 张娟. 过渡金属催化吲哚的串联去芳构化反应研究进展[J]. 有机化学, 2023, 43(4): 1271-1291. |
| [12] | 莫百川, 陈春霞, 彭进松. 木质素及其衍生物负载金属催化剂在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1215-1240. |
| [13] | 李靖鹏, 黄顺桃, 杨棋, 李伟强, 刘腾, 黄超. 利用连续流动技术合成(Z)-N-乙烯基取代N,O-缩醛[J]. 有机化学, 2023, 43(4): 1550-1558. |
| [14] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [15] | 纪健, 刘进华, 管丛, 陈绪文, 赵芸, 刘顺英. 原位生成的磺酸催化N-磺酰基-1,2,3-三氮唑与醇偶联高区域选择性合成N2-取代1,2,3-三氮唑[J]. 有机化学, 2023, 43(3): 1168-1176. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||