有机化学 ›› 2023, Vol. 43 ›› Issue (3): 974-999.DOI: 10.6023/cjoc202211022 上一篇 下一篇
所属专题: 中国女科学家专辑
综述与进展
王海清a, 杨爽a, 张宇辰a,*( ), 石枫a,b,*(
), 石枫a,b,*( )
)
收稿日期:2022-11-16
修回日期:2023-01-17
发布日期:2023-02-06
通讯作者:
张宇辰, 石枫
作者简介:基金资助:
Haiqing Wanga, Shuang Yanga, Yuchen Zhanga( ), Feng Shia,b(
), Feng Shia,b( )
)
Received:2022-11-16
Revised:2023-01-17
Published:2023-02-06
Contact:
Yuchen Zhang, Feng Shi
About author:Supported by:文章分享
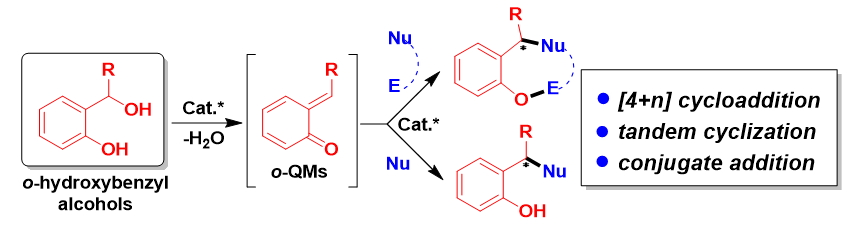
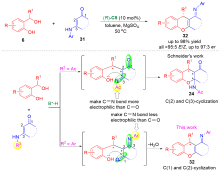
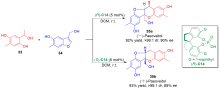
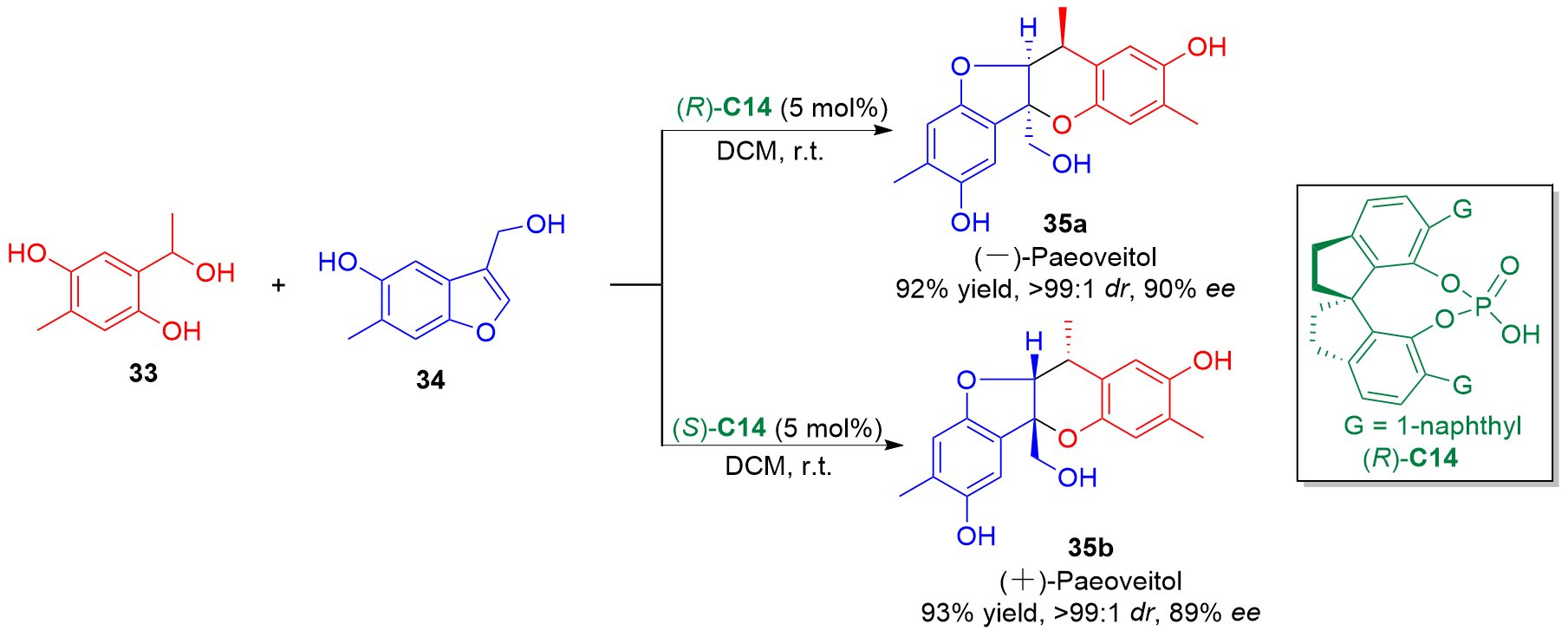
邻亚甲基苯醌(o-QMs)是一类高反应活性的有机中间体, 在碳-碳键及碳-杂原子键的催化不对称构筑中发挥着重要作用. 但是, 该类中间体高度不稳定, 导致这类中间体参与的催化不对称反应发展缓慢. 利用前体原位产生o-QMs中间体可以有效解决这一问题. 因此, 发展原位产生的o-QMs中间体参与的催化不对称反应引起了化学工作者的关注. 而发展此类反应的关键是开发简便易得、结构稳定的o-QMs中间体前体. 邻羟基苄醇是一类具有独特优势的o-QMs前体, 近年来, 其参与的催化不对称[4+n]环加成反应、串联环化反应及亲核加成反应发展十分迅速, 已经成为合成手性含氧杂环及芳基甲烷衍生物的高效策略. 综述了邻羟基苄醇参与的催化不对称反应, 将为设计新型邻羟基苄醇及其参与的催化不对称反应提供新的思路.
王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999.
Haiqing Wang, Shuang Yang, Yuchen Zhang, Feng Shi. Advances in Catalytic Asymmetric Reactions Involving o-Hydroxybenzyl Alcohols[J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 974-999.



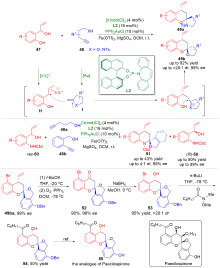

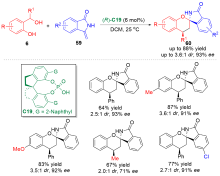
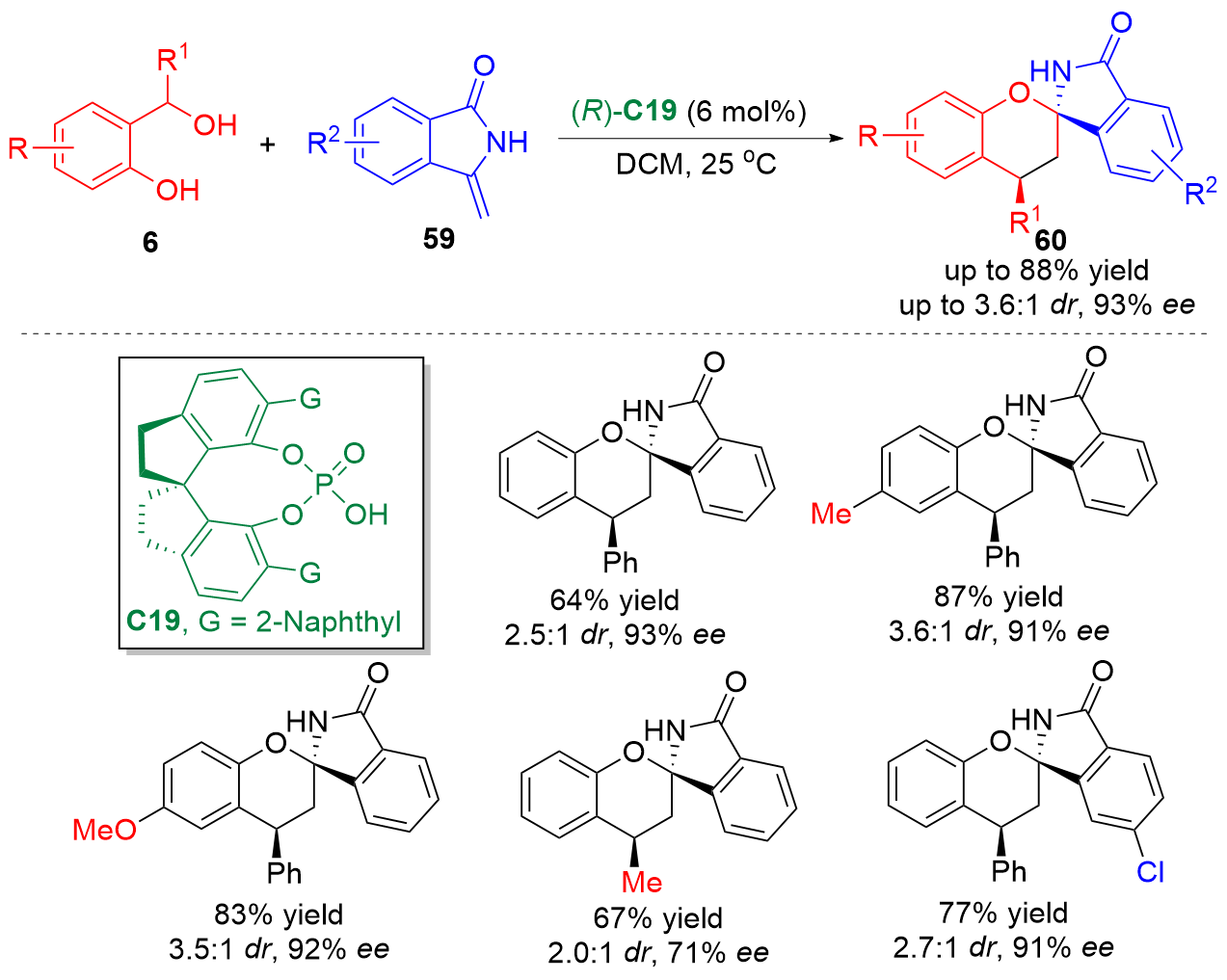
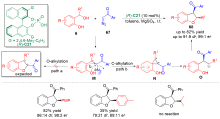
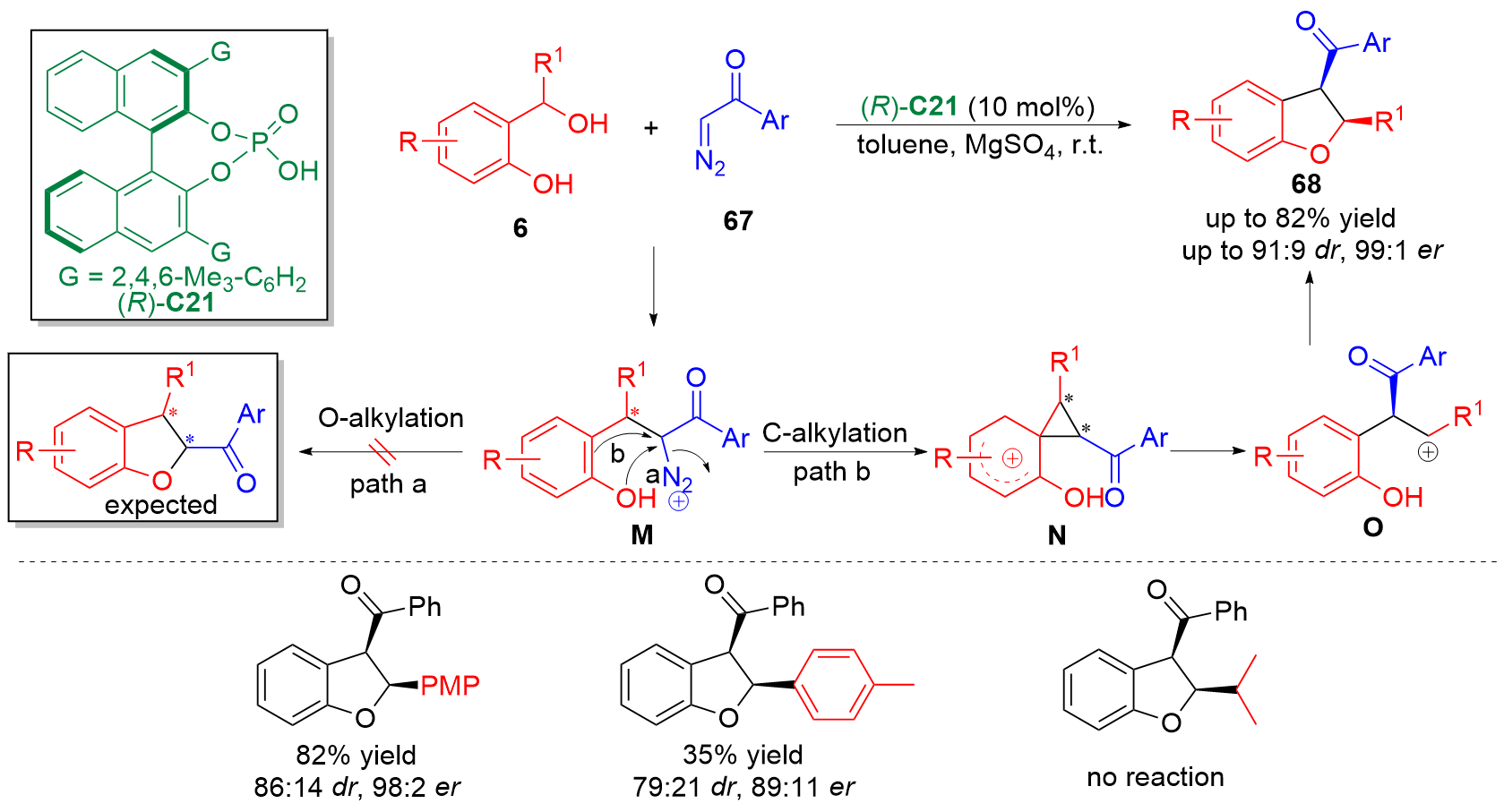
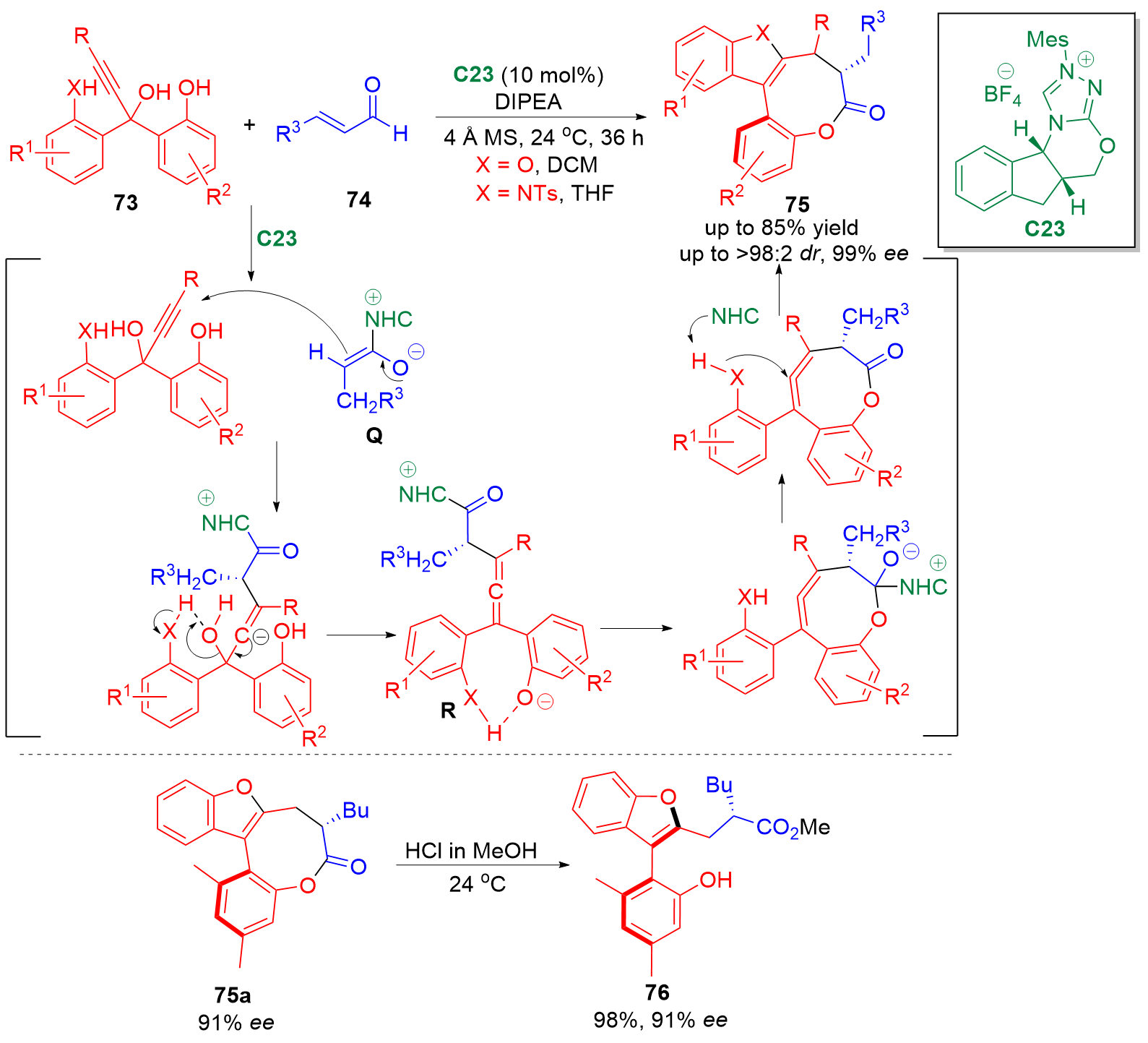

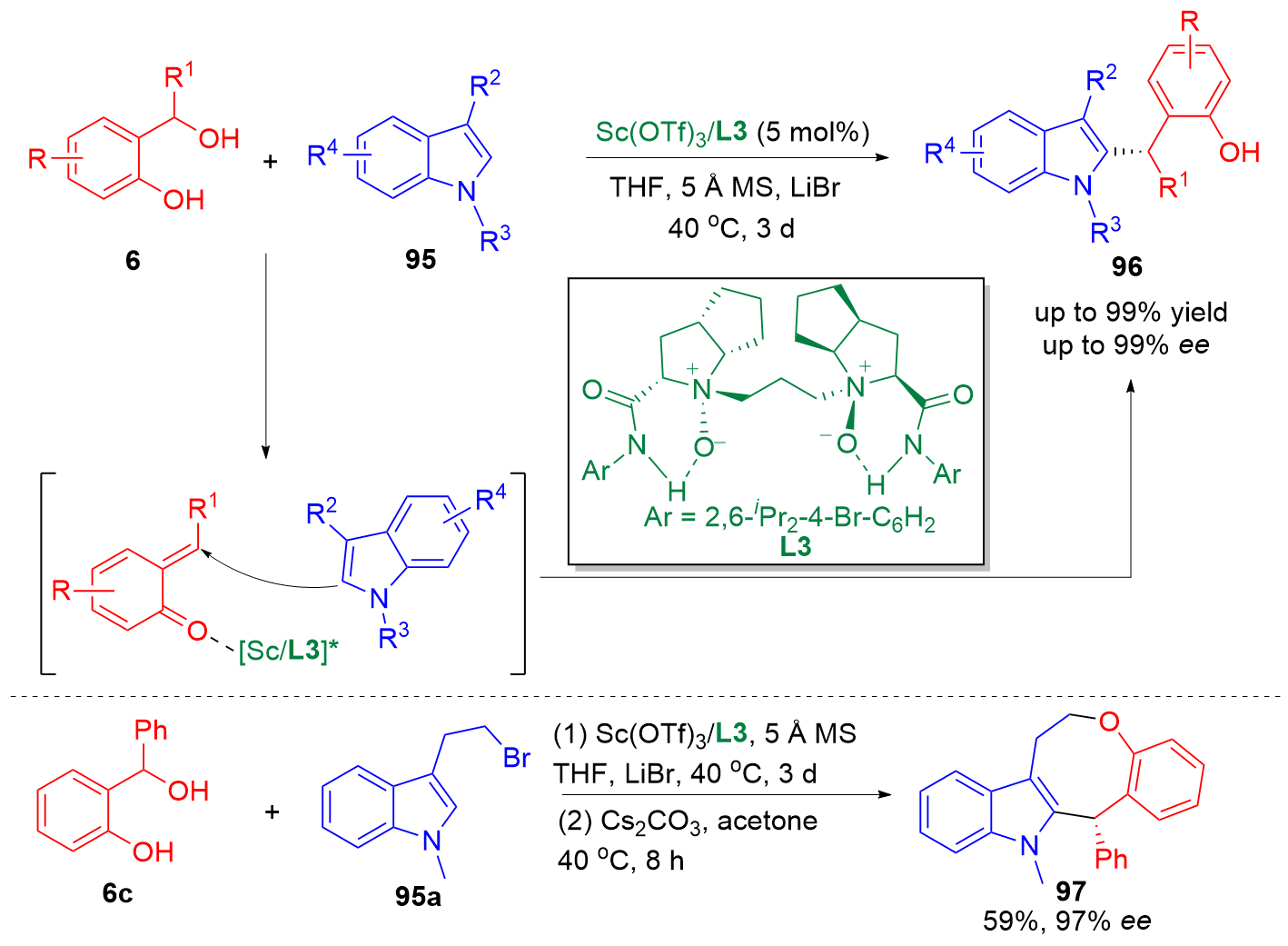
| [1] |
For an early report on o-QMs: (a) Fries, K.; Kann, K. Liebigs Ann. Chem. 1907, 353, 335.
|
|
For early reviews on o-QMs: (b) Water, R. W. V. D.; Pettus, T. R. R. Tetrahedron 2002, 58, 5367.
doi: 10.1016/S0040-4020(02)00496-9 |
|
|
(c) Ferreira, S. B.; da Silva, F. Pinto, A. C.; Gonzaga, D. T. G.; Ferreira, V. F. J. Heterocycl. Chem. 2009, 46, 1080.
doi: 10.1002/jhet.v46:6 |
|
|
(d) Yoshida, H.; Ohshita, J.; Kunai, A. Bull. Chem. Soc. Jpn. 2010, 83, 199.
doi: 10.1246/bcsj.20090245 |
|
| [2] |
For reviews on racemic reactions involving o-QMs: (a) Singh, M. S.; Nagaraju, A.; Anand, N.; Chowdhury, S. RSC Adv. 2014, 4, 55924.
doi: 10.1039/C4RA11444B |
|
(b) Bai, W.-J.; David, J. G.; Feng, Z.-G.; Weaver, M. G.; Wu, K.-L.; Pettus, T. R. R. Acc. Chem. Res. 2014, 47, 3655.
doi: 10.1021/ar500330x |
|
|
(c) Osipov, D. V.; Osyanin, V. A.; Klimochkin, Y. N. Russ. Chem. Rev. 2017, 86, 625.
doi: 10.1070/RCR4679 |
|
|
(d) Barta, P.; Fülöp, F.; Szatmári, I. Beilstein J. Org. Chem. 2018, 14, 560.
|
|
| [3] |
For early reviews on catalytic asymmetric reaction involving o-QMs: (a) Pathak, T. P.; Sigman, M. S. J. Org. Chem. 2011, 76, 9210.
doi: 10.1021/jo201789k pmid: 27513764 |
|
(b) Wang, Z.; Sun, J. Synthesis 2015, 47, 3629.
doi: 10.1055/s-00000084 pmid: 27513764 |
|
|
(c) Caruana, L.; Fochi, M.; Bernardi, L. Molecules 2015, 20, 11733.
doi: 10.3390/molecules200711733 pmid: 27513764 |
|
|
(d) Jaworski, A. A.; Scheidt, K. A. J. Org. Chem. 2016, 81, 10145.
pmid: 27513764 |
|
| [4] |
For recent reviews on o-QMs: (a) Mukhopadhyay, S.; Gharui, C.; Pan, S. C. Asian J. Org. Chem. 2019, 8, 1970.
doi: 10.1002/ajoc.v8.11 |
|
(b) Ma, Y.-H.; He, X.-Y.; Yang, Q.-Q.; Boucherif, A.; Xuan, J. Asian J. Org. Chem. 2021, 10, 1233.
doi: 10.1002/ajoc.v10.6 |
|
|
(c) Dorsch, C.; Schneider, C. Synthesis 2022, 54, 3125.
doi: 10.1055/a-1781-6538 |
|
|
(d) Li, X.; Li, Z.; Sun, J. Nat. Synth. 2022, 1, 426.
doi: 10.1038/s44160-022-00072-x |
|
| [5] |
For reviews on application of o-QMs in the total synthesis of natural products: (a) Willis, N. J.; Bray, C. D. Chem.-Eur. J. 2012, 18, 9160.
doi: 10.1002/chem.201200619 pmid: 22707392 |
|
(b) Ai, W.; Liao, D.; Lei, X. Chin. J. Org. Chem. 2015, 35, 1615. (in Chinese)
doi: 10.6023/cjoc201504031 pmid: 22707392 |
|
|
(艾文英, 廖道红, 雷晓光, 有机化学, 2015, 35, 1615.)
doi: 10.6023/cjoc201504031 pmid: 22707392 |
|
|
(c) Yang, B.; Gao, S. Chem. Soc. Rev. 2018, 47, 7926.
doi: 10.1039/C8CS00274F pmid: 22707392 |
|
| [6] |
Rueping, M.; Uria, U.; Lin, M.-Y.; Atodiresei, I. J. Am. Chem. Soc. 2011, 133, 3732.
doi: 10.1021/ja110213t pmid: 21355548 |
| [7] |
Wilcke, D.; Herdtweck, E.; Bach, T. Synlett 2011, 1235.
|
| [8] |
El-Sepelgy, O.; Haseloff, S.; Alamsetti, S. K.; Schneider, C. Angew. Chem., Int. Ed. 2014, 53, 7923.
doi: 10.1002/anie.201403573 pmid: 24938645 |
| [9] |
Hsiao, C.-C.; Liao, H.-H.; Rueping, M. Angew. Chem., Int. Ed. 2014, 53, 13258.
doi: 10.1002/anie.201406587 |
| [10] |
For some reviews: (a) Chen, D.-F.; Han, Z.-Y.; Zhou, X.-L.; Gong, L.-Z. Acc. Chem. Res. 2014, 47, 2365.
doi: 10.1021/ar500101a |
|
(b) Wang, P.-S.; Chen, D.-F.; Gong, L.-Z. Top. Curr. Chem. 2020, 378, 9.
|
|
|
(c) Wang, P.-S.; Gong, L.-Z. Acc. Chem. Res. 2020, 53, 2841.
doi: 10.1021/acs.accounts.0c00477 |
|
|
(d) Chen, D.-F.; Gong, L.-Z. J. Am. Chem. Soc. 2022, 144, 2415.
doi: 10.1021/jacs.1c11408 |
|
| [11] |
Alamsetti, S. K.; Spanka, M.; Schneider, C. Angew. Chem., Int. Ed. 2016, 55, 2392.
doi: 10.1002/anie.201509247 pmid: 26762542 |
| [12] |
(a) Yu, X.-Y.; Chen, J.-R.; Wei, Q.; Cheng, H.-G.; Liu, Z.-C.; Xiao, W.-J. Chem.-Eur. J. 2016, 22, 6774.
doi: 10.1002/chem.201601227 pmid: 26990670 |
|
(b) Zhang, Y.-C.; Zhu, Q.-N.; Yang, X.; Zhou, L.-J.; Shi, F. J. Org. Chem. 2016, 81, 1681.
doi: 10.1021/acs.joc.6b00078 pmid: 26990670 |
|
| [13] |
Jeong, H. J.; Kim, D. Y. Org. Lett. 2018, 20, 2944.
doi: 10.1021/acs.orglett.8b00993 pmid: 29715043 |
| [14] |
Spanka, M.; Schneider, C. Org. Lett. 2018, 20, 4769.
doi: 10.1021/acs.orglett.8b01865 |
| [15] |
Gçricke, F.; Schneider, C. Angew. Chem., Int. Ed. 2018, 57, 14736.
doi: 10.1002/anie.201809692 |
| [16] |
(a) Saha, S.; Schneider, C. Chem.-Eur. J. 2015, 21, 2348.
doi: 10.1002/chem.201406044 |
|
(b) Saha, S.; Schneider, C. Org. Lett. 2015, 17, 648.
doi: 10.1021/ol503662g |
|
| [17] |
For some reviews: (a) Mei, G.-J.; Shi, F. Synlett 2016, 27, 2515.
doi: 10.1055/s-0036-1588611 |
|
(b) Zhang, Y.-C.; Jang, F.; Shi, F. Acc. Chem. Res. 2020, 53, 425.
doi: 10.1021/acs.accounts.9b00549 |
|
|
(c) Tu, M.-S.; Chen, K.-W.; Wu, P.; Zhang, Y.-C.; Liu, X.-Q.; Shi, F. Org. Chem. Front. 2021, 8, 2643.
doi: 10.1039/D0QO01643H |
|
| [18] |
Zhao, J.-J.; Sun, S.-B.; He, S.-H.; Wu, Q.; Shi, F. Angew. Chem., Int. Ed. 2015, 54, 5460.
doi: 10.1002/anie.201500215 |
| [19] |
(a) Hsiao, C.-C.; Raja, S.; Liao, H.-H.; Atodiresei, I.; Rueping, M. Angew. Chem., Int. Ed. 2015, 54, 5762.
doi: 10.1002/anie.201409850 |
|
(b) Wu, Q.; Zhao, J.; Sun, S.; Tu, M.; Shi, F. Acta Chim. Sinica 2016, 74, 576. (in Chinese)
doi: 10.6023/A16020080 |
|
|
(吴琼, 赵佳佳, 孙斯兵, 屠蔓苏, 石枫, 化学学报, 2016, 74, 576.)
doi: 10.6023/A16020080 |
|
| [20] |
Zhao, J.-J.; Zhang, Y.-C.; Xu, M.-M.; Tang, M.; Shi, F. J. Org. Chem. 2015, 80, 10016.
doi: 10.1021/acs.joc.5b01613 |
| [21] |
Gharui, C.; Singh, S.; Pan, S. C. Org. Biomol. Chem. 2017, 15, 7272.
doi: 10.1039/C7OB01766A |
| [22] |
Li, T.-Z.; Geng, C.-A.; Yin, X.-J.; Yang, T.-H.; Chen, X.-L.; Huang, X.-Y.; Ma, Y.-B.; Zhang, X.-M.; Chen, J.-J. Org. Lett. 2017, 19, 429.
doi: 10.1021/acs.orglett.6b03801 |
| [23] |
Wang, Z.; Sun, J. Org. Lett. 2017, 19, 2334.
doi: 10.1021/acs.orglett.7b00867 |
| [24] |
Zhang, H.; Liu, G.; Guan, X.; Gao, J.; Qin, X.; Jiang, G.; Sun, D.; Zhang, G.; Zhang, S. Eur. J. Org. Chem. 2019, 2019, 7264.
doi: 10.1002/ejoc.201901286 |
| [25] |
(a) You, Y.; Li, T.-T.; Yuan, S.-P.; Xie, K.-X.; Wang, Z.-H.; Zhao, J.-Q.; Zhou, M.-Q.; Yuan, W.-C. Chem. Commun. 2020, 56, 439.
doi: 10.1039/C9CC08316B |
|
(b) Feng, S.; Yang, B.; Chen, T.; Wang, R.; Deng, Y.-H.; Shao, Z. J. Org. Chem. 2020, 85, 5231.
doi: 10.1021/acs.joc.9b03302 |
|
| [26] |
Ukis, R.; Schneider, C. J. Org. Chem. 2019, 84, 7175.
doi: 10.1021/acs.joc.9b00860 |
| [27] |
Lin, X.; Liu, X.; Wang, K.; Li, Q.; Liu, Y.; Li, C. Nat. Commun. 2021, 12, 4958.
doi: 10.1038/s41467-021-25198-y |
| [28] |
For some recent reviews: (a) Wu, Y.; Huo, X.; Zhang, W. Chem.- Eur. J. 2020, 26, 4895.
doi: 10.1002/chem.v26.22 |
|
(b) Kim, U. B.; Jung, D. J.; Jeon, H. J.; Rathwell, K.; Lee, S.-G. Chem. Rev. 2020, 120, 13382.
doi: 10.1021/acs.chemrev.0c00245 |
|
| [29] |
For some recent reviews: (a) Mei, G.-J.; Shi, F. Chem. Commun. 2018, 54, 6607.
doi: 10.1039/C8CC02364F |
|
(b) Xu, P.-W.; Yu, J.-S.; Chen, C.; Cao, Z.-Y.; Zhou, F.; Zhou, J. ACS Catal. 2019, 9, 1820.
doi: 10.1021/acscatal.8b03694 |
|
|
(c) Boddy, A. J.; Bull, J. A. Org. Chem. Front. 2021, 8, 1026.
doi: 10.1039/D0QO01085E |
|
|
(d) Wang, Y.; Cobo, A. A.; Franz, A. K. Org. Chem. Front. 2021, 8, 4315.
doi: 10.1039/D1QO00220A |
|
| [30] |
For some recent reviews: (a) Niu, B.; Wei, Y.; Shi, M. Org. Chem. Front. 2021, 8, 3475.
doi: 10.1039/D1QO00273B |
|
(b) Wang, J.; Blaszczyk, S. A.; Li, X.; Tang, W. Chem. Rev. 2021, 121, 110.
doi: 10.1021/acs.chemrev.0c00160 |
|
|
(c) Zhang, M.-M.; Qu, B.-L.; Shi, B.; Xiao, W.-J.; Lu, L.-Q. Chem. Soc. Rev. 2022, 51, 4146.
doi: 10.1039/D1CS00897H |
|
|
(d) You, Y.; Li, Q.; Zhang, Y.-P.; Zhao, J.-Q.; Wang, Z.-H.; Yuan, W.-C. ChemCatChem 2022, 14, e202101887.
|
|
|
(e) Zhang, J.; Chen, Y.; Wang, Q.; Shen, J.; Liu, Y.; Deng, W. Chin. J. Org. Chem. 2022, 42, 3051. (in Chinese)
doi: 10.6023/cjoc202206028 |
|
|
(张键, 陈颖, 汪全南, 沈佳欢, 刘飏子, 邓卫平, 有机化学, 2022, 42, 3051.)
doi: 10.6023/cjoc202206028 |
|
| [31] |
Yang, W.-L.; Shang, X.-Y.; Luo, X.; Deng, W.-P. Angew. Chem., Int. Ed. 2022, 61, e202203661.
|
| [32] |
Yang, W.-L.; Shang, X.-Y.; Ni, T.; Yan, H.; Luo, X.; Zheng, H.; Li, Z.; Deng, W.-P. Angew. Chem., Int. Ed. 2022, 61, e202210207.
|
| [33] |
Wang, T.; Huang, B.; Wang, Y.-Q. Adv. Synth. Catal. 2022, 364, 2596.
doi: 10.1002/adsc.v364.15 |
| [34] |
For a highlight: (a) Tan, W.; Shi, F., Chem. Synth. 2022, 2, 11.
doi: 10.20517/cs |
|
For a review: (b) Tan, W.; Zhang, J.-Y.; Gao, C.-H.; Shi, F. Sci. China: Chem. 2023, DOI: 10.1007/s11426-022-1471-2.
doi: 10.1007/s11426-022-1471-2 |
|
| [35] |
(a) Sun, M.; Ma, C.; Zhou, S.-J.; Lou, S.-F.; Xiao, J.; Jiao, Y.; Shi, F. Angew. Chem., Int. Ed. 2019, 58, 8703.
doi: 10.1002/anie.v58.26 |
|
For some reviews: (b) Mei, G.-J.; Shi, F. J. Org. Chem. 2017, 82, 7695.
doi: 10.1021/acs.joc.7b01458 |
|
|
(c) Petrini, M. Adv. Synth. Catal. 2020, 362, 1214.
doi: 10.1002/adsc.v362.6 |
|
|
(d) Zhang, H.-H.; Shi, F. Chin. J. Org. Chem. 2022, 42, 3351. (in Chinese)
doi: 10.6023/cjoc202203018 |
|
|
(张洪浩, 石枫, 有机化学, 2022, 42, 3351.)
doi: 10.6023/cjoc202203018 |
|
|
(e) Li, T.-Z.; Liu, S.-J.; Sun, Y.-W.; Deng, S.; Tan, W.; Jiao, Y.; Zhang, Y.-C.; Shi, F. Angew. Chem., Int. Ed. 2021, 60, 2355.
doi: 10.1002/anie.v60.5 |
|
|
(f) Yang, S.; Wang, H.-Q.; Gao, J.-N.; Tan, W.-X.; Zhang, Y.-C.; Shi, F. Eur. J. Org. Chem. 2022, 2022, e202200878.
|
|
|
(g) Shi, Y.-C.; Yan, X.-Y.; Wu, P.; Jiang, S.; Xu, R.; Tan, W.; Shi, F. Chin. J. Chem. 2023, 41, 27.
doi: 10.1002/cjoc.v41.1 |
|
| [36] |
Suneja, A.; Loui, H. J.; Schneider, C. Angew. Chem., Int. Ed. 2020, 59, 5536.
doi: 10.1002/anie.201913603 pmid: 31895488 |
| [37] |
Suneja, A.; Schneider, C. Org. Lett. 2018, 20, 7576.
doi: 10.1021/acs.orglett.8b03311 pmid: 30407018 |
| [38] |
Pandit, R. P.; Kim, S. T.; Ryu, D. H. Angew. Chem., Int. Ed. 2019, 58, 13427.
doi: 10.1002/anie.v58.38 |
| [39] |
Kim, S. T.; Pandit, R. P.; Yun, J.; Ryu, D. H. Org. Lett. 2021, 23, 213.
doi: 10.1021/acs.orglett.0c03937 |
| [40] |
Lu, S.; Ong, J.-Y.; Yang, H.; Poh, S. B.; Liew, X.; Seow, C. S. D.; Wong, M. W.; Zhao, Y. J. Am. Chem. Soc. 2019, 141, 17062.
doi: 10.1021/jacs.9b08510 |
| [41] |
Yang, W.-L.; Wang, Y.-L.; Li, W.; Gu, B.-M.; Wang, S.-W.; Luo, X.; Tian, B.-X.; Deng, W.-P. ACS Catal. 2021, 11, 12557.
doi: 10.1021/acscatal.1c03711 |
| [42] |
For recent reviews on six-membered axially chiral scaffolds: (a) Wang, Y.-B.; Tan, B. Acc. Chem. Res. 2018, 51, 534.
doi: 10.1021/acs.accounts.7b00602 pmid: 33491680 |
|
(b) Liao, G.; Zhou, T.; Yao, Q.-J.; Shi, B.-F. Chem. Commun. 2019, 55, 8514.
doi: 10.1039/C9CC03967H pmid: 33491680 |
|
|
(c) Carmona, J. A.; Rodríguez-Franco, C.; Fernández, R.; Hornillos, V.; Lassaletta, J. M. Chem. Soc. Rev. 2021, 50, 2968.
doi: 10.1039/d0cs00870b pmid: 33491680 |
|
|
(d) Cheng, J. K.; Xiang, S.-H.; Li, S.; Ye, L.; Tan, B. Chem. Rev. 2021, 121, 4805.
doi: 10.1021/acs.chemrev.0c01306 pmid: 33491680 |
|
|
(e) Liu, C.-X.; Zhang, W.-W.; Yin, S.-Y.; Gu, Q.; You, S.-L. J. Am. Chem. Soc. 2021, 143, 14025.
doi: 10.1021/jacs.1c07635 pmid: 33491680 |
|
|
(f) Wu, Y.-J.; Liao, G.; Shi, B.-F. Green Synth. Catal. 2022, 3, 117.
pmid: 33491680 |
|
|
(g) Mei, G.-J.; Koay, W. L.; Guan, C.-Y.; Lu, Y. Chem 2022, 8, 1855.
doi: 10.1016/j.chempr.2022.04.011 pmid: 33491680 |
|
|
(h) Zhang, X.; Zhao, K.; Gu, Z. Acc. Chem. Res. 2022, 55, 1620.
doi: 10.1021/acs.accounts.2c00175 pmid: 33491680 |
|
|
(i) Bai, X.-F.; Cui, Y.-M.; Cao, J.; Xu, L.-W. Acc. Chem. Res. 2022, 55, 2545.
doi: 10.1021/acs.accounts.2c00417 pmid: 33491680 |
|
| [43] |
For recent reviews on five-membered axially chiral scaffolds: (a) Bonne, D.; Rodriguez, J. Chem. Commun. 2017, 53, 12385.
doi: 10.1039/C7CC06863H |
|
(b) Bonne, D.; Rodriguez, J. Eur. J. Org. Chem. 2018, 2018, 2417.
doi: 10.1002/ejoc.201800078 |
|
|
(c) Zhang, S.; Liao, G.; Shi, B. Chin. J. Org. Chem. 2019, 39, 1522. (in Chinese)
doi: 10.6023/cjoc201904030 |
|
|
(张硕, 廖港, 史炳锋, 有机化学, 2019, 39, 1522.)
doi: 10.6023/cjoc201904030 |
|
|
(d) Li, T.-Z.; Liu, S.-J.; Tan, W.; Shi, F. Chem.-Eur. J. 2020, 26, 15779.
doi: 10.1002/chem.v26.68 |
|
|
(e) Zhang, H.-H.; Shi, F. Acc. Chem. Res. 2022, 55, 2562.
doi: 10.1021/acs.accounts.2c00465 |
|
|
(f) Cheng, J.-K.; Xiang, S.-H.; Tan, B. Acc. Chem. Res. 2022, 55, 2920.
doi: 10.1021/acs.accounts.2c00509 |
|
|
(g) Qin, W.; Liu, Y.; Yan, H. Acc. Chem. Res. 2022, 55, 2780.
doi: 10.1021/acs.accounts.2c00486 |
|
|
For a recent example: (h) Sheng, F.-T.; Yang, S.; Wu, S.-F.; Zhang, Y.-C.; Shi, F. Chin. J. Chem. 2022, 40, 2151.
doi: 10.1002/cjoc.v40.18 |
|
| [44] |
Gou, B.-B.; Tang, Y.; Lin, Y.-H.; Yu, L.; Jian, Q.-S.; Sun, H.-R.; Chen, J.; Zhou, L. Angew. Chem., Int. Ed. 2022, 61, e202208174.
|
| [45] |
For a review: Kshatriya, R.; Jejurkar, V. P.; Saha, S. Eur. J. Org. Chem. 2019, 2019, 3818.
doi: 10.1002/ejoc.201900465 |
| [46] |
Zhao, W.; Wang, Z.; Chu, B.; Sun, J. Angew. Chem., Int. Ed. 2015, 54, 1910.
doi: 10.1002/anie.201405252 |
| [47] |
Saha, S.; Alamsetti, S. K.; Schneider, C. Chem. Commun. 2015, 51, 1461.
doi: 10.1039/C4CC08559K |
| [48] |
Wu, Q.; Ma, C.; Du, X.-H.; Chen, Y.; Huang, T.-Z.; Shi, X.-Q.; Tu, S.-J.; Cai, P.-J. Tetrahedron: Asymmetry 2016, 27, 307.
doi: 10.1016/j.tetasy.2016.03.002 |
| [49] |
Wu, J.-L.; Wang, J.-Y.; Wu, P.; Mei, G.-J.; Shi, F. Org. Chem. Front. 2017, 4, 2465.
doi: 10.1039/C7QO00649G |
| [50] |
Zheng, J.; Lin, L.; Dai, L.; Yuan, X.; Liu, X.; Feng, X. Chem.-Eur. J. 2016, 22, 18254.
doi: 10.1002/chem.201604088 |
| [51] |
For some examples: (a) Qi, L.-W.; Mao, J.-H.; Zhang, J.; Tan, B. Nat. Chem. 2018, 10, 58.
doi: 10.1038/nchem.2866 pmid: 31508869 |
|
(b) Ma, C.; Jiang, F.; Sheng, F.-T.; Jiao, Y.; Mei, G.-J.; Shi, F. Angew. Chem., Int. Ed. 2019, 58, 3014.
doi: 10.1002/anie.v58.10 pmid: 31508869 |
|
|
(c) Hu, Y.-L.; Wang, Z.; Yang, H.; Chen, J.; Wu, Z.-B.; Lei, Y.; Zhou, L. Chem. Sci. 2019, 10, 6777.
doi: 10.1039/C9SC00810A pmid: 31508869 |
|
|
(d) Tian, M.; Bai, D.; Zheng, G.; Chang, J.; Li, X. J. Am. Chem. Soc. 2019, 141, 9527.
doi: 10.1021/jacs.9b04711 pmid: 31508869 |
|
|
(e) Wang, L.; Zhong, J.; Lin, X. Angew. Chem., Int. Ed. 2019, 58, 15824.
doi: 10.1002/anie.201909855 pmid: 31508869 |
|
|
(f) Peng, L.; Li, K.; Xie, C.; Li, S.; Xu, D.; Qin, W.; Yan, H. Angew. Chem., Int. Ed. 2019, 58, 17199.
doi: 10.1002/anie.201908961 pmid: 31508869 |
|
|
(g) Wang, C.-S.; Li, T.-Z.; Liu, S.-J.; Zhang, Y.-C.; Deng, S.; Jiao, Y.; Shi, F. Chin. J. Chem. 2020, 38, 543.
doi: 10.1002/cjoc.v38.6 pmid: 31508869 |
|
|
(h) Ding, W.-Y.; Yu, P.; An, Q.-J.; Bay, K. L.; Xiang, S.-H.; Li, S.; Chen, Y.; Houk, K. N.; Tan, B. Chem 2020, 6, 2046.
doi: 10.1016/j.chempr.2020.06.001 pmid: 31508869 |
|
| [52] |
Zhang, H.-H.; Wang, C.-S.; Li, C.; Mei, G.-J.; Li, Y.; Shi, F. Angew. Chem., Int. Ed. 2017, 56, 116.
doi: 10.1002/anie.201608150 |
| [53] |
Jiang, F.; Chen, K.-W.; Wu, P.; Zhang, Y.-C.; Jiao, Y.; Shi, F. Angew. Chem., Int. Ed. 2019, 58, 15104.
doi: 10.1002/anie.201908279 pmid: 31441203 |
| [54] |
For some recent examples: (a) Mao, J.-H.; Wang, Y.-B.; Yang, L.; Xiang, S.-H.; Wu, Q.-H.; Cui, Y.; Lu, Q.; Lv, J.; Li, S.; Tan, B. Nat. Chem. 2021, 13, 982.
doi: 10.1038/s41557-021-00750-x |
|
(b) Wang, J.-Y.; Sun, M.; Yu, X.-Y.; Zhang, Y.-C.; Tan, W.; Shi, F. Chin. J. Chem. 2021, 39, 2163.
doi: 10.1002/cjoc.v39.8 |
|
|
(c) Wang, C.-S.; Wei, L.; Fu, C.; Wang, X.-H.; Wang, C.-J. Org. Lett. 2021, 23, 7401.
doi: 10.1021/acs.orglett.1c02574 |
|
|
(d) Mi, R.; Chen, H.; Zhou, X.; Li, N.; Ji, D.; Wang, F.; Lan, Y.; Li, X. Angew. Chem., Int. Ed. 2022, 61, e202111860.
|
|
|
(e) Wu, P.; Yu, L.; Gao, C.-H.; Cheng, Q.; Deng, S.; Jiao, Y.; Tan, W.; Shi, F. Fund. Res. 2022, DOI: 10.1016/j.fmre.2022.01.002.
doi: 10.1016/j.fmre.2022.01.002 |
|
|
(f) Chen, K.-W.; Chen, Z.-H.; Yang, S.; Wu, S.-F.; Zhang, Y.-C.; Shi, F. Angew. Chem., Int. Ed. 2022, 61, e202116829.
|
|
|
(g) Hang, Q.-Q.; Wu, S.-F.; Yang, S.; Wang, X.; Zhong, Z.; Zhang, Y.-C.; Shi, F. Sci. China: Chem. 2022, 65, 1929.
doi: 10.1007/s11426-022-1363-y |
|
| [55] |
(a) Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed., Wiley Blackwell, New York, 2010.
|
|
(b) Gandini, A. In Polymer Chemistry, Springer, Berlin, 1977.
|
|
| [56] |
Cho, Y. S.; Kim, S. T.; Ryu, D. H. Org. Lett. 2022, 24, 1732.
doi: 10.1021/acs.orglett.2c00404 |
| [57] |
Lai, Z.; Wang, Z.; Sun, J. Org. Lett. 2015, 17, 6058.
doi: 10.1021/acs.orglett.5b03072 |
| [58] |
Lai, Z.; Sun, J. Synlett 2016, 27, 555.
doi: 10.1055/s-00000083 |
| [59] |
Chen, M.; Han, Y.; Ma, D.; Wang, Y.; Lai, Z.; Sun, J. Chin. J. Chem. 2018, 36, 587.
doi: 10.1002/cjoc.v36.7 |
| [60] |
Xu, M.-M.; Wang, H.-Q.; Wan, Y.; He, G.; Yan, J.; Zhang, S.; Wang, S.-L.; Shi, F. Org. Chem. Front. 2017, 4, 358.
doi: 10.1039/C6QO00549G |
| [1] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [2] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [3] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [4] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [5] | 李梦竹, 孟博莹, 兰文捷, 傅滨. 邻亚甲醌与硫叶立德反应合成2,3-二取代苯并二氢呋喃化合物[J]. 有机化学, 2024, 44(1): 195-203. |
| [6] | 马虎, 黄丹凤, 王克虎, 唐朵朵, 冯杨, 任园园, 王君娇, 胡雨来. 3-(三氟甲基)吡唑类化合物的合成[J]. 有机化学, 2023, 43(9): 3257-3267. |
| [7] | 程春霞, 吴露平, 沙风, 伍新燕. 手性叔膦-酰胺不对称催化香豆素与Morita-Baylis-Hillman碳酸酯之间的插烯烯丙基烷基化反应[J]. 有机化学, 2023, 43(9): 3188-3195. |
| [8] | 陈祖佳, 宇世伟, 周永军, 李焕清, 邱琪雯, 李妙欣, 汪朝阳. BF3•OEt2作为催化剂与合成子在有机合成中的应用进展[J]. 有机化学, 2023, 43(9): 3107-3118. |
| [9] | 陈祖良, 魏颖静, 张俊良. 供体-受体氮杂环丙烷碳-碳键断裂的环加成反应研究进展[J]. 有机化学, 2023, 43(9): 3078-3088. |
| [10] | 王熠, 张键, 刘飏子, 罗晓燕, 邓卫平. 钯催化不对称[3+4]环加成构建吲哚并环庚烷[J]. 有机化学, 2023, 43(8): 2864-2877. |
| [11] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [12] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [13] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [14] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [15] | 罗诚, 尹艳丽, 江智勇. P-手性膦氧化物的不对称合成研究进展[J]. 有机化学, 2023, 43(6): 1963-1976. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||