有机化学 ›› 2023, Vol. 43 ›› Issue (4): 1241-1270.DOI: 10.6023/cjoc202209003 上一篇 下一篇
综述与进展
收稿日期:2022-09-03
修回日期:2022-10-13
发布日期:2022-11-15
通讯作者:
应安国
基金资助:
Linsheng Bai, Peng Hong, Anguo Ying( )
)
Received:2022-09-03
Revised:2022-10-13
Published:2022-11-15
Contact:
Anguo Ying
Supported by:文章分享

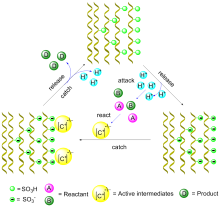
聚丙烯腈纤维(PANF)具有出色的机械强度、耐化学性和良好的热稳定性, 而且易于进行改性. 在聚丙烯腈纺丝原液中加入添加剂或功能单体, 或对PANF进行热处理等方法可以实现物理改性. 而PANF的化学表面改性包括胺化、酰胺化、氧化、还原、交联、水解、酸处理和化学枝接等方法, 表面改性给PANF带来许多特殊官能团, 使其可以进一步被用作有机物、有机配体、酶以及过渡金属的载体. 通过负载催化活性位点从而得到具有催化活性的功能化PANF. 近年功能化PANF作为非均相催化剂被广泛应用于有机合成领域, 综述了功能化PANF催化剂在有机反应中的研究成果与进展, 包括缩合反应、偶联反应、加成反应、氧化还原反应及多组分一锅法反应等. 介绍了纤维催化剂的合成及结构, 讨论了催化性能, 分析了可能的催化机理, 为开发更优的功能化PANF做铺垫.
白林盛, 洪鹏, 应安国. 功能化聚丙烯腈纤维促进有机反应的研究进展[J]. 有机化学, 2023, 43(4): 1241-1270.
Linsheng Bai, Peng Hong, Anguo Ying. Research Progress of Functional Polyacrylonitrile Fiber in Promoting Organic Reaction[J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1241-1270.
| Entry | Fiber | Retention strength/cN | Retention of breaking strengtha/% |
|---|---|---|---|
| 1 | PANF | 11.0 | 100 |
| 2 | C-PANF-Na | 8.1 | 73 |
| 3 | C-PANF-Na-1 | 8.1 | 73 |
| 4 | C-PANF-Na-18 | 7.9 | 72 |
| Entry | Fiber | Retention strength/cN | Retention of breaking strengtha/% |
|---|---|---|---|
| 1 | PANF | 11.0 | 100 |
| 2 | C-PANF-Na | 8.1 | 73 |
| 3 | C-PANF-Na-1 | 8.1 | 73 |
| 4 | C-PANF-Na-18 | 7.9 | 72 |
| Entry | Catalyst | Catalyst amount/mol% | Rection condition | Yielda/% | Reuse time | Ref |
|---|---|---|---|---|---|---|
| 1 | AOFs-Pd(0) | 0.8 | DMF, 110 ℃, 3 h | 95.5 | 3 | [ |
| 2 | Pd(PNODAM-5) | 2.0 | Heptane, 100 ℃, 22 h | 96 | 3 | [ |
| 3 | Pd/APS-MIL-101 | 0.93 | DMF, 120 ℃, 1 h | 97 | — | [ |
| 4 | FeO3O4@PCA/Pd(0)-b-PEG | 0.05 | H2O, 90 ℃, 2 h | 98 | 10 | [ |
| 5 | PdNPore | 2.0 | MeOH, 80 ℃, 18 h | 94 | 5 | [ |
| 6 | Pd-MPTAT-1 | 0.97 | H2O/EtOH (V∶V=1∶1), reflux, 6 h | 95 | 2 | [ |
| 7 | Pd-SP-CMP | 0.6 | 1,4-Dioxane/H2O (V∶V=1∶1), 80 ℃, 12 h | 99 | — | [ |
| 8 | Pd@IPN | 0.0229 | H2O, 100 ℃, 12 h | 96 | 11 | [ |
| 9 | PANPhenF-Pd(0) | 0.1 | Solvent-free, 110 ℃, 3 h | 97 | 6 | [ |
| Entry | Catalyst | Catalyst amount/mol% | Rection condition | Yielda/% | Reuse time | Ref |
|---|---|---|---|---|---|---|
| 1 | AOFs-Pd(0) | 0.8 | DMF, 110 ℃, 3 h | 95.5 | 3 | [ |
| 2 | Pd(PNODAM-5) | 2.0 | Heptane, 100 ℃, 22 h | 96 | 3 | [ |
| 3 | Pd/APS-MIL-101 | 0.93 | DMF, 120 ℃, 1 h | 97 | — | [ |
| 4 | FeO3O4@PCA/Pd(0)-b-PEG | 0.05 | H2O, 90 ℃, 2 h | 98 | 10 | [ |
| 5 | PdNPore | 2.0 | MeOH, 80 ℃, 18 h | 94 | 5 | [ |
| 6 | Pd-MPTAT-1 | 0.97 | H2O/EtOH (V∶V=1∶1), reflux, 6 h | 95 | 2 | [ |
| 7 | Pd-SP-CMP | 0.6 | 1,4-Dioxane/H2O (V∶V=1∶1), 80 ℃, 12 h | 99 | — | [ |
| 8 | Pd@IPN | 0.0229 | H2O, 100 ℃, 12 h | 96 | 11 | [ |
| 9 | PANPhenF-Pd(0) | 0.1 | Solvent-free, 110 ℃, 3 h | 97 | 6 | [ |
| Entry | Catalyst | Rection condition | Yielda/% | Reuse time | Ref. |
|---|---|---|---|---|---|
| 1 | SiO2-NHC-Cu | MeOH, 50 ℃, 3 h | 90 | 6 | [ |
| 2 | Ps-Cu | MeOH, 40 ℃, 10 h | 99 | 5 | [ |
| 3 | Cu@PI-COF | MeOH-H2O, r.t., 8 h | 87 | 8 | [ |
| 4 | Cu-(tpa)MOF | EtOH, Et3N, r.t., 12 h | 99 | 5 | [ |
| 5 | Cu3(BTC)2 | EtOH, Et3N, 60 ℃, 12 h | 92 | 4 | [ |
| 6 | Cu-doped CoFe2O4 | MeOH, Et3N, r.t., 5 h | 92 | 7 | [ |
| 7 | URJC-1-MOF | DMF, K2CO3, r.t., 15 h | 61 | 5 | [ |
| 8 | CuO NPs | MeOH-H2O, r.t., 10 h | 88 | 6 | [ |
| 9 | CuCl2@PANPA-2F | MeOH, 60 ℃, 3 h | 98 | 5 | [ |
| Entry | Catalyst | Rection condition | Yielda/% | Reuse time | Ref. |
|---|---|---|---|---|---|
| 1 | SiO2-NHC-Cu | MeOH, 50 ℃, 3 h | 90 | 6 | [ |
| 2 | Ps-Cu | MeOH, 40 ℃, 10 h | 99 | 5 | [ |
| 3 | Cu@PI-COF | MeOH-H2O, r.t., 8 h | 87 | 8 | [ |
| 4 | Cu-(tpa)MOF | EtOH, Et3N, r.t., 12 h | 99 | 5 | [ |
| 5 | Cu3(BTC)2 | EtOH, Et3N, 60 ℃, 12 h | 92 | 4 | [ |
| 6 | Cu-doped CoFe2O4 | MeOH, Et3N, r.t., 5 h | 92 | 7 | [ |
| 7 | URJC-1-MOF | DMF, K2CO3, r.t., 15 h | 61 | 5 | [ |
| 8 | CuO NPs | MeOH-H2O, r.t., 10 h | 88 | 6 | [ |
| 9 | CuCl2@PANPA-2F | MeOH, 60 ℃, 3 h | 98 | 5 | [ |
| Entry | Fiber | Retention strength/cN | Retention of breaking strengtha/% |
|---|---|---|---|
| 1 | PANF | 10.56 | 100 |
| 2 | PANF-CEIMBr | 7.93 | 75.1 |
| 3 | PANF-CEIMBr-1 | 7.91 | 74.9 |
| 4 | PANF-CEIMBr-21 | 7.64 | 72.3 |
| Entry | Fiber | Retention strength/cN | Retention of breaking strengtha/% |
|---|---|---|---|
| 1 | PANF | 10.56 | 100 |
| 2 | PANF-CEIMBr | 7.93 | 75.1 |
| 3 | PANF-CEIMBr-1 | 7.91 | 74.9 |
| 4 | PANF-CEIMBr-21 | 7.64 | 72.3 |

| Entry | Active center species | Catalyst |
|---|---|---|
| 1 | Tertiary amine group | 1a~1b, 5, 8, 16, 48, 52a~52b |
| 2 | Secondary amine group | 6 |
| 3 | Proline amides | 3, 12 |
| 4 | Aminopyridine group | 7, 10, 33, 38b~38d, 46a~46b, 47 |
| 5 | Chiral pyrrolidine | 34 |
| 6 | Phosphoric acid group | 45, 51 |
| 7 | Proline group | 4, 11 |
| 8 | L-Lysine | 9 |
| 9 | Sulfonic acid group | 17 |
| 10 | Phosphotungstic acid | 4, 50 |
| 11 | Quaternary ammonium group | 13, 14, 35, 37, 41, 49 |
| 12 | Imidazole ionic liquid | 36a~36e |
| 13 | Supported metal Cu | 21, 28, 30, 31, 39, 53 |
| 14 | Pd nanoparticles | 19, 23 |
| 15 | Pd complex | 20, 22 |
| 16 | Doping Pd | 25 |
| 17 | Ag complex | 26 |
| 18 | Ag nanoparticles | 32 |
| 19 | Supported metal Au | 27, 42 |
| 20 | Ni complex | 29 |
| 21 | Fe complex | 44, 54 |
| Entry | Active center species | Catalyst |
|---|---|---|
| 1 | Tertiary amine group | 1a~1b, 5, 8, 16, 48, 52a~52b |
| 2 | Secondary amine group | 6 |
| 3 | Proline amides | 3, 12 |
| 4 | Aminopyridine group | 7, 10, 33, 38b~38d, 46a~46b, 47 |
| 5 | Chiral pyrrolidine | 34 |
| 6 | Phosphoric acid group | 45, 51 |
| 7 | Proline group | 4, 11 |
| 8 | L-Lysine | 9 |
| 9 | Sulfonic acid group | 17 |
| 10 | Phosphotungstic acid | 4, 50 |
| 11 | Quaternary ammonium group | 13, 14, 35, 37, 41, 49 |
| 12 | Imidazole ionic liquid | 36a~36e |
| 13 | Supported metal Cu | 21, 28, 30, 31, 39, 53 |
| 14 | Pd nanoparticles | 19, 23 |
| 15 | Pd complex | 20, 22 |
| 16 | Doping Pd | 25 |
| 17 | Ag complex | 26 |
| 18 | Ag nanoparticles | 32 |
| 19 | Supported metal Au | 27, 42 |
| 20 | Ni complex | 29 |
| 21 | Fe complex | 44, 54 |
| [1] |
Xu, G.; Wang, L.; Li, M. M.; Tao, M. L.; Zhang, W. Q. Green Chem. 2017, 19, 5818.
doi: 10.1039/C7GC02935G |
| [2] |
Cole-Hamilton, D. J. Science 2003, 299, 1702.
pmid: 12637737 |
| [3] |
Wang, X. L.; Yang, M.; Zhu, L. J.; Zhu, X. N.; Wang, S. R. J. Fuel Chem. Technol. 2020, 48, 456.
doi: 10.1016/S1872-5813(20)30020-7 |
| [4] |
Gogoi, P.; Dutta, A. K.; Saikia, S.; Borah, R. Appl. Catal., A 2016, 523, 321.
doi: 10.1016/j.apcata.2016.06.015 |
| [5] |
Schulze, J. S.; Migenda, J.; Becker, M.; Schuler, S. M.; Wende, R. C.; Schreiner, P. R.; Smarsly, B. M. J. Mater. Chem. A 2020, 8, 4107.
doi: 10.1039/C9TA12416K |
| [6] |
Liu, G. H.; Zong, Z. M.; Liu, Z. Q.; Liu, F. J.; Zhang, Y. Y.; Wei, X. Y. Fuel Process Technol. 2018, 179, 114.
doi: 10.1016/j.fuproc.2018.05.035 |
| [7] |
Pan, S.; Yan, S.; Osako, T.; Uozumi, Y. ACS Sustainable Chem. Eng. 2017, 5, 10722.
doi: 10.1021/acssuschemeng.7b02646 |
| [8] |
Baran, T. J. Mol. Struct. 2017, 1141, 535.
doi: 10.1016/j.molstruc.2017.03.122 |
| [9] |
Lu, X. T.; Li, S. N.; Wang, L. M.; Huang, S. J.; Liu, Z. Q.; Liu, Y. J.; Ying, A. G. Fuel 2022, 310, 122318.
doi: 10.1016/j.fuel.2021.122318 |
| [10] |
Badoga, S.; Sohani, K.; Zheng, Y.; Dalai, A. K. Fuel Process. Technol. 2017, 168, 140.
doi: 10.1016/j.fuproc.2017.08.033 |
| [11] |
McNamara, C. A.; Dixon, M. J.; Bradley, M. Chem. Rev. 2002, 102, 3275.
doi: 10.1021/cr0103571 |
| [12] |
Zheng, Y. W.; Zhao, W.; Jia, D.; Cui, L.; Liu, J. Q. Chem. Eng. J. 2019, 364, 70.
doi: 10.1016/j.cej.2019.01.076 |
| [13] |
Zhang, Y. Y.; Sun, Y. L.; Peng, L. F.; Yang, J. Q.; Jia, H. H.; Zhang, Z. R.; Shan, B.; Xie, J. Energy Storage Mater. 2019, 21, 287.
|
| [14] |
Xiao, J.; Wang, L.; Ran, J. R.; Zhao, J. Y.; Tao, M. L.; Zhang, W. Q. React. Funct. Polym. 2020, 146, 104394.
doi: 10.1016/j.reactfunctpolym.2019.104394 |
| [15] |
Xu, G.; Xu, W. S.; Tian, S.; Zheng, W. J.; Yang, T.; Wu, Y. X.; Xiong, Q. Z.; Kalkhajeh, Y. K.; Gao, H. J. Chem. Eng. J. 2021, 416, 127889.
doi: 10.1016/j.cej.2020.127889 |
| [16] |
Xing, X. L.; Yang, H. X.; Tao, M. L.; Zhang, W. Q. J. Hazard. Mater. 2015, 297, 207.
doi: 10.1016/j.jhazmat.2015.05.001 |
| [17] |
Li, Y.; Abedalwafa, M. A.; Ni, C. F.; Sanbhal, N.; Wang, L. React. Funct. Polym. 2019, 138, 18.
doi: 10.1016/j.reactfunctpolym.2019.02.009 |
| [18] |
Hong, P.; Wang, L. M.; Bai, L. S.; Liu, Z. Q.; Liu, Y. J.; Yang J. G., Ying, A. G. Dyes Pigm. 2022, 197, 109902.
doi: 10.1016/j.dyepig.2021.109902 |
| [19] |
Liu, X. M.; Li, M. Y.; Han, G. Y.; Dong, J. H. Electrochim. Acta 2010, 55, 2983.
doi: 10.1016/j.electacta.2010.01.014 |
| [20] |
Shi, X. L.; Xing, X. L.; Lin, H. K.; Zhang, W. Q. Adv. Synth. Catal. 2014, 356, 2349.
doi: 10.1002/adsc.v356.10 |
| [21] |
Xu, C. Z.; Du, J. G.; Ma, L. C.; Li, G. W.; Tao, M. L.; Zhang, W. Q. Tetrahedron 2013, 69, 4749.
doi: 10.1016/j.tet.2013.02.084 |
| [22] |
Liu, R. X.; Zhang, B. W.; Tang, H. X. React. Funct. Polym. 1999, 39, 71.
doi: 10.1016/S1381-5148(97)00174-0 |
| [23] |
Moroi, G.; Bilba, D.; Bilba, N. Polym. Degrad. Stab. 2004, 84, 207.
doi: 10.1016/j.polymdegradstab.2003.10.013 |
| [24] |
Xiao, J.; Xu, G.; Wang, L.; Li, P. Y.; Zhang, W. Q.; Ma, N.; Tao, M. L. J. Ind. Eng. Chem. 2019, 77, 65.
doi: 10.1016/j.jiec.2019.04.001 |
| [25] |
Li, G. W.; Xiao, J.; Zhang, W. Q. Dyes Pigm. 2012, 92, 1091.
doi: 10.1016/j.dyepig.2011.08.015 |
| [26] |
Zheng, L. S.; Li, P. Y.; Tao, M. L.; Zhang, W. Q. Catal. Commun. 2019, 118, 19.
doi: 10.1016/j.catcom.2018.09.009 |
| [27] |
Polander, L. E.; Barlow, S.; Seifried, B. M.; Marder, S. R. J. Org. Chem. 2012, 77, 9426.
doi: 10.1021/jo301876v pmid: 23030064 |
| [28] |
Kudirka, R. A.; Barfield, R. M.; McFarland, J. M.; Drake, P. M.; Carlson, A.; Bañas, S.; Zmolek, W.; Garofalo, A. W.; Rabuka, D. ACS Med. Chem. Lett. 2016, 7, 994.
pmid: 27882197 |
| [29] |
Liang, F. S.; Pu, Y. J.; Kurata, T.; Kido, J. J.; Nishide, H. Polymer 2005, 46, 3767.
doi: 10.1016/j.polymer.2005.03.036 |
| [30] |
Zhu, H.; Xu, G.; Du, H. M.; Zhang, C. L.; Ma, N.; Zhang, W. Q. J. Catal. 2019, 374, 217.
doi: 10.1016/j.jcat.2019.04.040 |
| [31] |
Li, G. W.; Xiao, J.; Zhang, W. Q. Chin. Chem. Lett. 2013, 24, 52.
doi: 10.1016/j.cclet.2012.12.007 |
| [32] |
Li, G. W.; Xiao, J.; Zhang, W. Q. Green Chem. 2011, 13, 1828.
doi: 10.1039/c0gc00877j |
| [33] |
Li, G. W.; Xiao, J.; Zhang, W. Q. Green Chem. 2012, 14, 2234.
doi: 10.1039/c2gc35483g |
| [34] |
Xu, G.; Jin, M. C.; Kalkhajeh, Y. K.; Wang, L.; Tao, M. L.; Zhang, W. Q. J. Cleaner Prod. 2019, 231, 77.
doi: 10.1016/j.jclepro.2019.05.211 |
| [35] |
Hu, Q. Q.; Shi, X. L.; Chen, Y. J.; Han, X. F.; Duan, P. G.; Zhang, W. Q. J. Ind. Eng. Chem. 2017, 54, 75.
doi: 10.1016/j.jiec.2017.05.020 |
| [36] |
Li, P. Y.; Liu, Y. Y.; Ma, N.; Zhang, W. Q. Catal. Lett. 2018, 148, 813.
doi: 10.1007/s10562-017-2287-y |
| [37] |
Li, P. Y.; Mi, L. W.; Liu, Y. Y.; Zhang, W. Q.; Shi, X. L. J. Ind. Eng. Chem. 2020, 81, 323.
doi: 10.1016/j.jiec.2019.09.021 |
| [38] |
Masamune, S.; Choy, W.; Petersen, J. S.; Sita, L. R. Angew. Chem., Int. Ed. 1985, 24, 1.
|
| [39] |
Liu, B. Y.; Zhao, D. S.; Xu, D. Q.; Xu, Z. Y. Chem. Res. Chin. Univ. 2007, 23, 549.
doi: 10.1016/S1005-9040(07)60120-2 |
| [40] |
Xiao, J.; Li, G. W.; Zhang, W. Q. Chem. Res. Chin. Univ. 2013, 29, 256.
doi: 10.1007/s40242-013-2236-2 |
| [41] |
Zhu, H.; Zhang, C. L.; Ma, N.; Tao, M. L.; Zhang, W. Q. Appl. Catal., A 2020, 608, 117842.
doi: 10.1016/j.apcata.2020.117842 |
| [42] |
Wang, L.; Xu, G.; Xiao, J.; Tao, M. L.; Zhang, W. Q. Ind. Eng. Chem. Res. 2019, 58, 12401.
doi: 10.1021/acs.iecr.9b01375 |
| [43] |
Li, P. Y.; Liu, Y. Y.; Wang, L.; Tao, M. L.; Zhang, W. Q. J. Appl. Polym. Sci. 2018, 135, 45992.
doi: 10.1002/app.45992 |
| [44] |
Zhang, Z.; Wang, M.; Zhang, C. F.; Zhang, Z. X.; Lu, J. M.; Wang, F. Chem. Commun. 2015, 51, 9205.
doi: 10.1039/C5CC02785C |
| [45] |
Zhou, J. G.; Fang, J. J. Org. Chem. 2011, 76, 7730.
doi: 10.1021/jo201054k |
| [46] |
Modi, A.; Ali, W.; Mohanta, P. R.; Khatun, N.; Patel, B. K. ACS Sustainable Chem. Eng. 2015, 3, 2582.
doi: 10.1021/acssuschemeng.5b00817 |
| [47] |
Laha, J. K.; Patel, K. V.; Tummalapalli, K. S.; Dayal, N. Chem. Commun. 2016, 52, 10245.
doi: 10.1039/C6CC04259G |
| [48] |
De Vries, J. G. Can. J. Chem. 2001, 79, 1086.
doi: 10.1139/v01-033 |
| [49] |
Madasu, S. B.; Vekariya, N. A.; Kiran, M. H.; Gupta, B.; Islam, A.; Douglas, P. S.; Babu, K. R. Beilstein J. Org. Chem. 2012, 8, 1400.
pmid: 23019477 |
| [50] |
Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Chem. Rev. 2018, 118, 2249.
doi: 10.1021/acs.chemrev.7b00443 |
| [51] |
Jagtap, S. Catalysts 2017, 7, 267.
doi: 10.3390/catal7090267 |
| [52] |
Mpungose, P. P.; Vundla, Z. P.; Maguire, G. E.; Friedrich, H. B. Molecules 2018, 23, 1676.
doi: 10.3390/molecules23071676 |
| [53] |
Xiao, J.; Wang, L.; Zhang, H. N.; Ma, N.; Tao, M. L.; Zhang, W. Q. Chem. Eng. Sci. 2022, 247, 117053.
doi: 10.1016/j.ces.2021.117053 |
| [54] |
Xiao, J.; Zhang, H. N.; Ejike, A. C.; Wang, L.; Tao, M. L.; Zhang, W. Q. React. Funct. Polym. 2021, 161, 104843.
doi: 10.1016/j.reactfunctpolym.2021.104843 |
| [55] |
Sruthi, P. R.; Anjali, S.; Varghese, N.; Anas, S. J. Organomet. Chem. 2020, 921, 121354.
doi: 10.1016/j.jorganchem.2020.121354 |
| [56] |
Sruthi, P. R.; Sarika, V.; Suku, A.; Krishnan, A.; Anas, S. Inorg. Chim. Acta 2020, 502, 119305.
doi: 10.1016/j.ica.2019.119305 |
| [57] |
Xing, G. Y. Fibers Polym. 2016, 17, 194.
doi: 10.1007/s12221-016-4778-7 |
| [58] |
Shao, L. J.; Liu., J.; Ye, Y. H.; Zhang, X. M.; Qi, C. Z. Appl. Organomet. Chem. 2011, 25, 699.
|
| [59] |
Shao, L. J.; Qi, C. Z. Appl. Catal., A 2013, 468, 26.
doi: 10.1016/j.apcata.2013.08.010 |
| [60] |
Shao, L. J.; Qi, C. Z. Fibers Polym. 2014, 15, 2233.
doi: 10.1007/s12221-014-2233-1 |
| [61] |
Guo, L. P.; Bai, J.; Wang, J. Z.; Liang, H. O.; Li, C. P.; Sun, W. Y.; Meng, Q. R. J. Mol. Catal. A: Chem. 2015, 400, 95.
doi: 10.1016/j.molcata.2015.02.009 |
| [62] |
Han, F. S. Chem. Soc. Rev. 2013, 42, 5270.
doi: 10.1039/c3cs35521g |
| [63] |
Zhong, L.; Chokkalingam, A.; Cha, W. S.; Lakhi, K. S.; Su, X. Y.; Lawrence, G.; Vinu, A. Catal. Today 2015, 243, 195.
doi: 10.1016/j.cattod.2014.08.038 |
| [64] |
Kundu, D.; Patra, A. K.; Sakamoto, J.; Uyama, H. React. Funct. Polym. 2014, 79, 8.
doi: 10.1016/j.reactfunctpolym.2014.03.002 |
| [65] |
Marziale, A. N.; Jantke, D.; Faul, S. H.; Reiner, T.; Herdtweck, E.; Eppinger, J. Green Chem. 2011, 13, 169.
doi: 10.1039/C0GC00522C |
| [66] |
Yu, D. D.; Bai, J.; Wang, J. Z.; Li, C. P. J. Inorg. Organomet. Polym. Mater. 2016, 26, 914.
doi: 10.1007/s10904-016-0384-9 |
| [67] |
Peshkov, V. A.; Pereshivko, O. P.; Van der Eycken, E. V. Chem. Soc. Rev. 2012, 41, 3790.
doi: 10.1039/c2cs15356d |
| [68] |
Huo, X.; Liu, J.; Wang, B. D.; Zhang, H. L.; Yang, Z. Y.; She, X. G.; Xi, P. X. J. Mater. Chem. A 2013, 1, 651.
doi: 10.1039/C2TA00485B |
| [69] |
Hashmi, A. S. K. Pure Appl. Chem. 2010, 82, 657.
doi: 10.1351/PAC-CON-09-10-17 |
| [70] |
Jaimes, M. C. B.; Rominger, F.; Pereira, M. M.; Carrilho, R. M. B.; Carabineiro, S. A. C.; Hashmi, A. S. K. Chem. Commun. 2014, 50, 4937.
doi: 10.1039/c4cc00839a |
| [71] |
Cao, J.; Xu, G.; Li, P. Y.; Tao, M. L.; Zhang, W. Q. ACS Sustainable Chem. Eng. 2017, 5, 3438.
doi: 10.1021/acssuschemeng.7b00103 |
| [72] |
Cao, J.; Li, P. Y.; Xu, G.; Tao, M. L.; Ma, N.; Zhang, W. Q. Chem. Eng. J. 2018, 349, 456.
doi: 10.1016/j.cej.2018.05.046 |
| [73] |
Hu, Q. Q.; Shi, X. L.; Chen, Y. J.; Wang, F.; Weng, Y. J.; Duan, P. G. J. Ind. Eng. Chem. 2019, 69, 387.
doi: 10.1016/j.jiec.2018.09.047 |
| [74] |
Shi, X. L.; Sun, B. Y.; Chen, Y. J.; Hu, Q. Q.; Li, P. Y.; Meng, Y. L.; Duan, P. G. J. Catal. 2019, 372, 321.
doi: 10.1016/j.jcat.2019.03.020 |
| [75] |
Trost, B. M.; Masters, J. T. Chem. Soc. Rev. 2016, 45, 2212.
doi: 10.1039/C5CS00892A |
| [76] |
Zhang, B.; Wang, Y.; Yang, S. P.; Zhou, Y.; Wu, W. B.; Tang, W.; Zuo, J. P.; Li, Y.; Yue, J. M. J. Am. Chem. Soc. 2012, 134, 20605.
doi: 10.1021/ja310482z pmid: 23214963 |
| [77] |
Gholami, M.; Tykwinski, R. R. Chem. Rev. 2006, 106, 4997.
doi: 10.1021/cr0505573 |
| [78] |
Eisler, S.; Slepkov, A. D.; Elliott, E.; Luu, T.; McDonald, R.; Hegmann, F. A.; Tykwinski, R. R. J. Am. Chem. Soc. 2005, 127, 2666.
pmid: 15725024 |
| [79] |
Shi, X. L.; Hu, Q. Q.; Wang, F.; Zhang, W. Q.; Duan, P. G. J. Catal. 2016, 337, 233.
doi: 10.1016/j.jcat.2016.01.022 |
| [80] |
Zhang, C. L.; Zhu, H.; Gang, K. Y.; Tao, M. L.; Ma, N.; Zhang, W. Q. React. Funct. Polym. 2021, 160, 104831.
doi: 10.1016/j.reactfunctpolym.2021.104831 |
| [81] |
Cao, J.; Tian, H. Q. Chem.-Asian J. 2018, 13, 1561.
doi: 10.1002/asia.v13.12 |
| [82] |
Sonogashira, K. J. Organomet. Chem. 2002, 653, 46.
doi: 10.1016/S0022-328X(02)01158-0 |
| [83] |
Paterson, I.; Davies, R. D. M.; Marquez, R. Angew. Chem., Int. Ed. 2001, 113, 623.
doi: 10.1002/(ISSN)1521-3757 |
| [84] |
Toyota, M.; Komori, C.; Ihara, M. J. Org. Chem. 2000, 65, 7110.
pmid: 11031036 |
| [85] |
Li, J. Z.; Ambroise, A.; Yang, S. I.; Diers, J. R.; Seth, J.; Wack, C. R.; Bocian, D. F.; Holten, D.; Lindsey, J. S. J. Am. Chem. Soc. 1999, 121, 8927.
doi: 10.1021/ja991730d |
| [86] |
Strachan, J. P.; Gentemann, S.; Seth, J.; Kalsbeck, W. A.; Lindsey, J. S.; Holten, D.; Bocian, D. F. Inorg. Chem. 1998, 37, 1191.
doi: 10.1021/ic970967c |
| [87] |
Wang, W. S.; Shangguan, S. H.; Qiu, N.; Hu, C. Q.; Zhang, L.; Hu, Y. Z. Bioorg. Med. Chem. 2013, 21, 2879.
doi: 10.1016/j.bmc.2013.03.061 |
| [88] |
Fu, T. L.; Wang, I. J. Dyes Pigm. 2008, 76, 590.
doi: 10.1016/j.dyepig.2007.02.007 |
| [89] |
Puterová, Z.; Romiszewski, J.; Mieczkowski, J.; Gorecka, E. Tetrahedron 2012, 68, 8172.
doi: 10.1016/j.tet.2012.07.075 |
| [90] |
Narlawar, R.; Lane, J. R.; Doddareddy, M.; Lin, J.; Brussee, J.; IJzerman, A. P. J. Med. Chem. 2010, 53, 3028.
doi: 10.1021/jm901252a |
| [91] |
Li, P. Y.; Du, J. G.; Xie, Y. J.; Tao, M. L.; Zhang, W. Q. ACS Sustainable Chem. Eng. 2016, 4, 1139.
doi: 10.1021/acssuschemeng.5b01216 |
| [92] |
Du, J. G.; Shuai, B.; Tao, M. L.; Wang, G. W.; Zhang, W. Q. Green Chem. 2016, 18, 2625.
doi: 10.1039/C5GC02622A |
| [93] |
Yuan, X. Y.; Du, H. M.; Zhao, J. Y.; Chima, A. E.; Ma, N.; Tao, M. L.; Zhang, W. Q. Catal. Lett. 2021, 151, 832.
doi: 10.1007/s10562-020-03340-7 |
| [94] |
Song, Q. W.; Zhou, Z. H.; He, L. N. Green Chem. 2017, 19, 3707.
doi: 10.1039/C7GC00199A |
| [95] |
Lang, X. D.; He, L. N. Chem. Rec. 2016, 16, 1337.
doi: 10.1002/tcr.201500293 |
| [96] |
Shi, X. L.; Chen, Y. J.; Duan, P. G.; Zhang, W. Q.; Hu, Q. Q. ACS Sustainable Chem. Eng. 2018, 6, 7119.
doi: 10.1021/acssuschemeng.8b01051 |
| [97] |
Geng, H.; Zhang, C. L.; Tao, M. L.; Ma, N.; Zhang, W. Q. J. CO2 Util. 2021, 49, 101559.
|
| [98] |
Li, P. Y.; Liu, Y. Y.; Mi, L. W.; Shi, X. L.; Duan, P. G.; Cao, J. L.; Zhang, W. Q. Catal. Today 2020, 355, 162.
doi: 10.1016/j.cattod.2019.06.049 |
| [99] |
Shi, X. L.; Chen, Y. J.; Hu, Q. Q.; Zhang, W. Q.; Luo, C. X.; Duan, P. G. J. Ind. Eng. Chem. 2017, 53, 134.
|
| [100] |
Liu, H.; Bai, J.; Wang, S.; Li, C. P.; Guo, L. P.; Liang, H. O.; Xu, T.; Sun, W. Y.; Li, H. Q. Colloids Surf., A 2014, 448, 154.
doi: 10.1016/j.colsurfa.2014.02.024 |
| [101] |
Du, J. G.; Xu, G.; Lin, H.; Wang, G. W.; Tao, M. L.; Zhang, W. Q. Green Chem. 2016, 18, 2726.
doi: 10.1039/C5GC02621K |
| [102] |
Arslan, O.; Eren, H.; Biyikli, N.; Uyar, T. ChemistrySelect 2017, 2, 8790.
doi: 10.1002/slct.201701329 |
| [103] |
Wang, M. L.; Jiang, T. T.; Lu, Y.; Liu, H. J.; Chen, Y. J. Mater. Chem. A 2013, 1, 5923.
doi: 10.1039/c3ta10293a |
| [104] |
Xiao, J.; Wu, Z. Y.; Li, K. L.; Zhao, Z. B.; Liu, C. Y. RSC Adv. 2022, 12, 1051.
doi: 10.1039/D1RA07321D |
| [105] |
Shi, X. L.; Chen, Y. J.; Hu, Q. Q.; Wang, F.; Duan, P. G. J. Ind. Eng. Chem. 2018, 60, 333.
doi: 10.1016/j.jiec.2017.11.019 |
| [106] |
Zhen, Y. Z.; Lin, H. K.; Wang, S. Y.; Tao, M. L. RSC Adv. 2014, 4, 26122.
doi: 10.1039/C4RA03985H |
| [107] |
Li, P. Y.; Yang, Y.; Mi, L. W.; Gao, K.; Tao, M. L.; Chen, W. H. Catal. Lett. 2021, 151, 2056.
doi: 10.1007/s10562-020-03443-1 |
| [108] |
Xie, Y. J.; Liu, X. X.; Tao, M. L. J. Chem. Educ. 2016, 93, 2074.
doi: 10.1021/acs.jchemed.5b00933 |
| [109] |
Shi, X. L.; Lin, H. K.; Li, P. Y.; Zhang, W. Q. ChemCatChem 2014, 6, 2947.
doi: 10.1002/cctc.v6.10 |
| [110] |
Xiao, J.; Wang, L.; Ran, J. R.; Zhao, J. Y.; Ma, N.; Tao, M. L.; Zhang, W. Q. J. Cleaner Prod. 2020, 274, 122473.
doi: 10.1016/j.jclepro.2020.122473 |
| [111] |
Xu, G.; Cao, J.; Zhao, Y. L.; Zheng, L. S.; Tao, M. L.; Zhang, W. Q. Chem.-Asian J. 2017, 12, 2565.
doi: 10.1002/asia.v12.19 |
| [112] |
Li, P. Y.; Liu, Y. Y.; Cao, J.; Tao, M. L.; Zhang, W. Q. ChemCatChem 2017, 9, 3725.
doi: 10.1002/cctc.201700515 |
| [113] |
Shi, X. L.; Sun, B. Y.; Hu, Q. Q.; Liu, K.; Li, P. Y.; Liu, B. Z. Chem. Eng. J. 2020, 395, 125084.
doi: 10.1016/j.cej.2020.125084 |
| [114] |
Shi, X. L.; Sun, B. Y.; Hu, Q. Q.; Chen, Y. J.; Duan, P. G. Green Chem. 2019, 21, 3573.
doi: 10.1039/C9GC00987F |
| [115] |
Wu, J.; Du, X. L.; Ma, J.; Zhang, Y. P.; Shi, Q. C.; Luo, L. J.; Song, B. A.; Yang, S.; Hu, D. Y. Green Chem. 2014, 16, 3210.
doi: 10.1039/C3GC42400F |
| [116] |
Chen, J. X.; Su, W. K.; Wu, H. Y.; Liu, M. C.; Jin, C. Green Chem. 2007, 9, 972.
doi: 10.1039/b700957g |
| [117] |
Wu, Z. C.; Wu, Q.; Chen, M.; Tao, T. X. Asian J. Chem. 2013, 25, 5783.
doi: 10.14233/ajchem |
| [118] |
Bergbreiter, D. E.; Osburn, P. L.; Frels, J. D. J. Am. Chem. Soc. 2001, 123, 5783.
|
| [119] |
Hwang, Y. K.; Hong, D. Y.; Chang, J. S.; Jhung, S. H.; Seo, Y. K.; Kim, J.; Vimont, A.; Daturi, M.; Serre, C.; Férey, G. Angew. Chem., Int. Ed. 2008, 120, 4212.
doi: 10.1002/(ISSN)1521-3757 |
| [120] |
Tabatabaei Rezaei, S. J.; Shamseddin, A.; Ramazani, A.; Mashhadi Malekzadeh, A.; Azimzadeh Asiabi, P. Appl. Organomet. Chem. 2017, 31, e3707.
|
| [121] |
Kaneko, T.; Tanaka, S.; Asao, N.; Yamamoto, Y.; Chen, M. W.; Zhang, W.; Inoue, A. Adv. Synth. Catal. 2011, 353, 2927.
doi: 10.1002/adsc.v353.16 |
| [122] |
Modak, A.; Mondal, J.; Sasidharan, M.; Bhaumik, A. Green Chem. 2011, 13, 1317.
doi: 10.1039/c1gc15045f |
| [123] |
Ju, P. Y.; Wu, S. J.; Su, Q.; Li, X. D.; Liu, Z. Q.; Li, G. H.; Wu, Q. L. J. Mater. Chem. 2019, 7, 2660.
|
| [124] |
Zhan, K.; You, H. H.; Liu, W. Y.; Lu, J.; Lu, P.; Dong, J. React. Funct. Polym. 2011, 71, 756.
doi: 10.1016/j.reactfunctpolym.2011.04.007 |
| [125] |
Liu, P.; Li, P. H.; Wang, L. Synth. Commun. 2012, 42, 2595.
doi: 10.1080/00397911.2011.563024 |
| [126] |
Islam, S. M.; Salam, N.; Mondal, P.; Roy, A. S.; Ghosh, K.; Tuhina, K. J. Mol. Catal. 2014, 387, 7.
doi: 10.1016/j.molcata.2014.02.007 |
| [127] |
Altman, R. A.; Koval, E. D.; Buchwald, S. L. J. Org. Chem. 2007, 72, 6190.
pmid: 17625886 |
| [128] |
Devarajan, N.; Suresh, P. ChemCatChem 2016, 8, 2953.
doi: 10.1002/cctc.201600480 |
| [129] |
Anbu, N.; Dhakshinamoorthy, A. J. Ind. Eng. 2018, 65, 120.
|
| [130] |
Dutta, M. M.; Phukan, P. Catal. Commun. 2018, 109, 38.
doi: 10.1016/j.catcom.2018.02.014 |
| [131] |
Muñoz, A.; Leo, P.; Orcajo, G.; Martínez, F.; Calleja, G. ChemCatChem 2019, 11, 3376.
doi: 10.1002/cctc.v11.15 |
| [132] |
Borah, R. K.; Raul, P. K.; Mahanta, A.; Shchukarev, A.; Mikkola, J. P.; Thakur, A. J. Synlett 2017, 28, 1177.
doi: 10.1055/s-0036-1588741 |
| [1] | 李洋, 董亚楠, 李跃辉. 经由N-硼基酰胺中间体的酰胺高效转化合成腈类化合物[J]. 有机化学, 2024, 44(2): 638-643. |
| [2] | 李思达, 崔鑫, 舒兴中, 吴立朋. 钛催化的烯烃制备1,1-二硼化合物[J]. 有机化学, 2024, 44(2): 631-637. |
| [3] | 邹发凯, 王能中, 姚辉, 王慧, 刘明国, 黄年玉. 1β-/3R-芳基硫代糖的区域与立体选择性合成[J]. 有机化学, 2024, 44(2): 593-604. |
| [4] | 刘继宇, 李圣玉, 陈款, 朱茵, 张元. 三苯胺功能化有序介孔聚合物作为无金属光催化剂用于二硫化物合成[J]. 有机化学, 2024, 44(2): 605-612. |
| [5] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [6] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [7] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [8] | 黄净, 杨毅华, 张占辉, 刘守信. 酰胺键的绿色高效构建方法与技术进展[J]. 有机化学, 2024, 44(2): 409-420. |
| [9] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [10] | 朱彦硕, 王红言, 舒朋华, 张克娜, 王琪琳. 烷氧自由基引发1,5-氢原子转移实现C(sp3)—H键官能团化的研究进展[J]. 有机化学, 2024, 44(1): 1-17. |
| [11] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [12] | 李梦竹, 孟博莹, 兰文捷, 傅滨. 邻亚甲醌与硫叶立德反应合成2,3-二取代苯并二氢呋喃化合物[J]. 有机化学, 2024, 44(1): 195-203. |
| [13] | 童红恩, 郭宏宇, 周荣. 可见光促进惰性碳-氢键对羰基的加成反应进展[J]. 有机化学, 2024, 44(1): 54-69. |
| [14] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [15] | 文思, 丁宇浩, 田青于, 葛进, 程国林. 铑(III)催化苯甲亚胺酸乙酯和CF3-亚胺氧锍叶立德C—H 活化/环化反应合成CF3-1H-苯并[de][1,8]萘吡啶[J]. 有机化学, 2024, 44(1): 291-300. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||