有机化学 ›› 2023, Vol. 43 ›› Issue (11): 3861-3875.DOI: 10.6023/cjoc202304025 上一篇 下一篇
综述与进展
收稿日期:2023-04-19
修回日期:2023-06-01
发布日期:2023-06-13
作者简介:基金资助:
Yunpeng Qia†, Dengkai Linb†, Liang-An Chenb( )
)
Received:2023-04-19
Revised:2023-06-01
Published:2023-06-13
Contact:
E-mail: About author:Supported by:文章分享
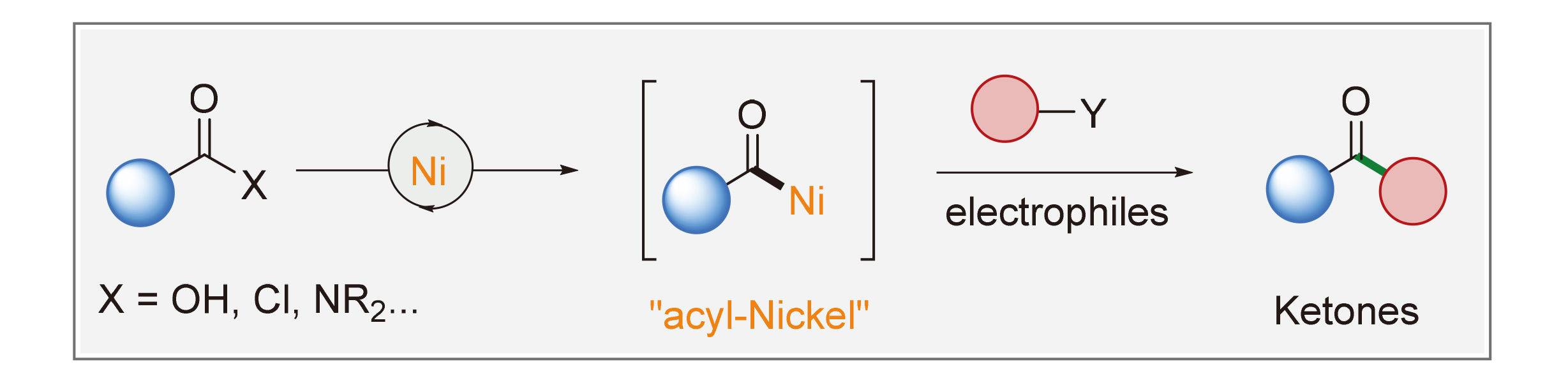
酰基镍是金属有机合成中的一类重要中间体, 近些年来, 以酰基镍为中间体的还原酰基化反应合成酮的策略引起了广泛地关注. 相较于金属亲核试剂参与的传统交叉偶联反应, 还原酰基化反应具有条件温和、步骤经济性高、官能团兼容性良好、环境友好等优点. 对近些年来镍催化羧酸或羧酸衍生物和各种亲电试剂的还原酰基化合成酮的最新研究进行了概述.
齐云鹏, 林登凯, 陈良安. 酰基镍作为关键中间体参与的酰基还原制备酮的研究进展[J]. 有机化学, 2023, 43(11): 3861-3875.
Yunpeng Qi, Dengkai Lin, Liang-An Chen. Research Progress on Reductive Acylation with Acyl-Ni as a Key Intermediate to Synthesize Ketones[J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3861-3875.

| [1] |
Battaglia, U.; Moody, C. J. Nat. Prod. 2010, 73, 1938.
doi: 10.1021/np100298m pmid: 20836523 |
| [2] |
Chong, Y. M.; Yin, W. F.; Ho, C. Y.; Mustafa, M. R.; Hadi, A. H. A.; Awang, K.; Narrima, P.; Koh, C.-L.; Appleton, D. R.; Chan, K.-G. J. Nat. Prod. 2011, 74, 2261.
doi: 10.1021/np100872k pmid: 21910441 |
| [3] |
Zhu, D. L.; Young, D. J.; Li, H. X. Synthesis 2020, 52, 3493.
doi: 10.1055/s-0040-1707183 |
| [4] |
Wotal, A. C.; Weix, D. J. Org. Lett. 2012, 14, 1476.
doi: 10.1021/ol300217x |
| [5] |
Wu, F.; Lu, W. B.; Qian, Q.; Ren, Q. H.; Gong, H. G. Org. Lett. 2012, 14, 3044.
doi: 10.1021/ol3011198 |
| [6] |
Lu, W.; Liang, Z.; Zhang, Y.; Wu, F.; Qian, Q.; Gong, H. G. Synthesis 2013, 45, 2234.
doi: 10.1055/s-00000084 |
| [7] |
Zhao, X.; Tu, H.-Y.; Guo, L.; Zhu, S.; Qing, F.-L.; Chu, L. Nat. Commun. 2018, 9, 3488.
doi: 10.1038/s41467-018-05951-6 |
| [8] |
Scattolin, T.; Deckers, K.; Schoenebeck, F. Org. Lett. 2017, 19, 5740.
doi: 10.1021/acs.orglett.7b02516 pmid: 29023131 |
| [9] |
Munoz, S. B.; Dang, H.; Ispizua-Rodriguez, X.; Mathew, T.; Prakash, G. K. S. Org. Lett. 2019, 21, 1659.
doi: 10.1021/acs.orglett.9b00197 |
| [10] |
Pan, F.-F.; Guo, P.; Li, C.-L.; Su, P.; Shu, X.-Z. Org. Lett. 2019, 21, 3701.
doi: 10.1021/acs.orglett.9b01164 |
| [11] |
Wang, J.; Hoerrner, M. E.; Watson, M. P.; Weix, D. J. Angew. Chem., Int. Ed. 2020, 59, 13484.
doi: 10.1002/anie.v59.32 |
| [12] |
Pulikottil, F. T.; Pilli, R.; Suku, R. V.; Rasappan, R. Org. Lett. 2020, 22, 2902.
doi: 10.1021/acs.orglett.0c00554 pmid: 32216317 |
| [13] |
Liao, J.; Basch, G. H.; Hoerrner, M. E.; Talley, M. R.; Boscoe, B. P.; Tucker, J. W.; Garnsey, M. R.; Waston, M. P. Org. Lett. 2019, 21, 2941.
doi: 10.1021/acs.orglett.9b01014 |
| [14] |
Cherney, A. H.; Kadunce, N. T.; Reisman, S. E. J. Am. Chem. Soc. 2013, 135, 7442.
doi: 10.1021/ja402922w pmid: 23634932 |
| [15] |
Lin, D.; Chen, Y.; Dong, Z.; Pei, P.; Ji, H.; Tai, L.; Chen, L.-A. CCS Chem. 2023, 5, 1386.
doi: 10.31635/ccschem.022.202202076 |
| [16] |
Wu, B.-B.; Xu, J.; Bian, K.-J.; Gao, Q.; Wang, X.-S. J. Am. Chem. Soc. 2022, 144, 6543.
doi: 10.1021/jacs.2c01422 |
| [17] |
Wu, J.; Wu, H.; Liu, X.; Zhang, Y.; Huang, G.; Zhang, C. Org. Lett. 2022, 24, 4322.
doi: 10.1021/acs.orglett.2c01208 |
| [18] |
Hoyos, P.; Sinisterra, J.-V.; Molinari, F.; Alcántara, A. R.; Domínguez De María, P. Acc. Chem. Res. 2010, 43, 288.
doi: 10.1021/ar900196n |
| [19] |
Jennings, L. K.; Robertson, L. P.; Rudolph, K. E.; Munn, A. L.; Carroll, A. R. J. Nat. Prod. 2019, 82, 2620.
doi: 10.1021/acs.jnatprod.9b00551 |
| [20] |
Ji, H.; Lin, D.; Tai, L.; Li, X.; Shi, Y.; Han, Q.; Chen, L.-A. J. Am. Chem. Soc. 2022, 144, 23019.
doi: 10.1021/jacs.2c10072 |
| [21] |
Gooßen, L. J.; Rodríguez, N.; Gooßen, K. Angew. Chem., Int. Ed. 2008, 47, 3100.
doi: 10.1002/anie.v47:17 |
| [22] |
Zhao, C.; Jia, X.; Wang, X.; Gong, H. J. Am. Chem. Soc. 2014, 136, 17645.
doi: 10.1021/ja510653n |
| [23] |
Ni, S.; Padial, N. M.; Kingston, C.; Vantourout, J. C.; Schmitt, D. C.; Edwards, J. C.; Kruszy, M. M.; Merchant, R. R.; Mykhailiuk, P. K.; Sanchez, B. B.; Yang, S.; Perry, M. A.; Gallego, G. M.; Mousseau, J. J.; Collins, M. R.; Cherney, R. J.; Lebed, P. S.; Chen, J. S.; Qin, T.; Baran, P. S. J. Am. Chem. Soc. 2019, 141, 6726.
doi: 10.1021/jacs.9b02238 |
| [24] |
Jiao, K.-J.; Ma, C.; Liu, D.; Qiu, H.; Cheng, B.; Mei, T.-S. Org. Chem. Front. 2021, 8, 6603.
doi: 10.1039/D1QO01219C |
| [25] |
Ruzi, R.; Liu, K.; Zhu, C.; Xie, J. Nat. Commun. 2020, 11, 3312.
doi: 10.1038/s41467-020-17224-2 |
| [26] |
Li, Y.; Shao, Q.; He, H.; Zhu, C.; Xue, X.-S.; Xie, J. Nat. Commun. 2022, 13, 10.
doi: 10.1038/s41467-021-27507-x |
| [27] |
Zhang, L.; Chen, S.; He, H.; Li, W.; Zhu, C.; Xie, J. Chem. Commun. 2021, 57, 9064.
doi: 10.1039/D1CC04188F |
| [28] |
He, J.; Song, P.; Xu, X.; Zhu, S.; Wang, Y. ACS Catal. 2019, 9, 3253.
doi: 10.1021/acscatal.9b00521 |
| [29] |
Jiang, X.; Sheng, F.-T.; Zhang, Y.; Deng, G.; Zhu, S. J. Am. Chem. Soc. 2022, 144, 21448.
doi: 10.1021/jacs.2c10785 |
| [30] |
Davidsen, S. K.; May, P. D.; Summers, J. B. J. Org. Chem. 1991, 56, 5482.
doi: 10.1021/jo00018a059 |
| [31] |
Hie, L.; Nathel, N. F. F.; Shah, T. K.; Baker, E. L.; Hong, X.; Yang, Y. F.; Liu, P.; Houk, K. N.; Garg, N. K. Nature 2015, 524, 79.
doi: 10.1038/nature14615 |
| [32] |
Simmons, B. J.; Weires, N. A.; Dander, J. E.; Garg, N. K. ACS Catal. 2016, 6, 3176.
doi: 10.1021/acscatal.6b00793 pmid: 32257581 |
| [33] |
Weires, N. A.; Baker, E. L.; Garg, N. K. Nat. Chem. 2016, 8, 75.
doi: 10.1038/nchem.2388 |
| [34] |
Hu, J.; Zhao, Y.; Liu, J.; Zhang, Y.; Shi, Z. Angew. Chem., Int. Ed. 2016, 55, 8718.
doi: 10.1002/anie.v55.30 |
| [35] |
Ni, S.; Zhang, W.; Mei, H.; Han, J.; Pan, Y. Org. Lett. 2017, 19, 2536.
doi: 10.1021/acs.orglett.7b00831 |
| [36] |
Yu, C.-G.; Matsuo, Y. Org. Lett. 2020, 22, 950.
doi: 10.1021/acs.orglett.9b04497 |
| [37] |
Zhuo, J.; Zhang, Y.; Li, Z.; Li, C. ACS Catal. 2020, 10, 3895.
doi: 10.1021/acscatal.0c00246 |
| [38] |
Kerackian, T.; Reina, A.; Bouyssi, D.; Monteiro, N.; Amgoune, A. Org. Lett. 2020, 22, 2240.
doi: 10.1021/acs.orglett.0c00442 pmid: 32148046 |
| [39] |
Kerackian, T.; Bouyssi, D.; Pilet, G.; Médebielle, M.; Monteiro, N.; Vantourout, J. C.; Amgoune, A. ACS Catal. 2022, 12, 12315.
doi: 10.1021/acscatal.2c03268 |
| [40] |
Ai, Y.; Ye, N.; Wang, Q.; Yahata, K.; Kishi, Y. Angew. Chem., Int. Ed. 2017, 56, 10791.
doi: 10.1002/anie.v56.36 |
| [41] |
Wu, X.; Han, J.; Xia, S.; Li, W.; Zhu, C.; Xie, J. CCS Chem. 2022, 4, 2469.
doi: 10.31635/ccschem.021.202101197 |
| [42] |
Yang, F.; Ding, D.; Wang, C. Org. Lett. 2020, 22, 9203.
doi: 10.1021/acs.orglett.0c03342 |
| [43] |
Xi, X.; Luo, Y.; Li, W.; Xu, M.; Zhao, H.; Chen, Y.; Zheng, S.; Qi, X.; Yuan, W. Angew. Chem., Int. Ed. 2022, 61, e202114731.
doi: 10.1002/anie.v61.3 |
| [44] |
Sun, Y.; Su, L.; Tong, W.; Yao, K.; Gong, H. Synlett 2021, 32, 1762.
doi: 10.1055/a-1550-7935 |
| [45] |
Xu, S.; Wang, K.; Kong, W. Org. Lett. 2019, 21, 7498.
doi: 10.1021/acs.orglett.9b02788 |
| [46] |
Shi, R.; Hu, X. Angew. Chem., Int. Ed. 2019, 58, 7454.
doi: 10.1002/anie.v58.22 |
| [47] |
Chen, H.; Yue, H.; Zhu, C.; Rueping, M. Angew. Chem., Int. Ed. 2022, e202204144.
|
| [48] |
Chen, J.; Zhu, S. J. Am. Chem. Soc. 2021, 143, 14089.
doi: 10.1021/jacs.1c07851 |
| [49] |
Chen, J.; Deng, G.; Wang, Y.; Zhu, S. Chin. J. Chem. 2023, 41, 294.
doi: 10.1002/cjoc.v41.3 |
| [50] |
Zheng, M.; Xue, W.; Xue, T.; Gong, H. Org. Lett. 2016, 18, 6152.
pmid: 27934381 |
| [51] |
Zhu, Z.; Gong, Y.; Tong, W.; Xue, W.; Gong, H. Org. Lett. 2021, 23, 2158.
doi: 10.1021/acs.orglett.1c00313 |
| [1] | 高宝昌, 石雨, 田媛, 张治国, 张婧如, 孙宇峰, 毛国梁, 戴凌燕. 4-甲基-2-氧代-6-芳氨基-二氢-吡喃-3-腈衍生物的合成[J]. 有机化学, 2024, 44(2): 644-649. |
| [2] | 王化坤, 任晓龙, 宣宜宁. 卤盐催化的α,β-环氧羧酸酯与异氰酸酯[3+2]环加成反应研究[J]. 有机化学, 2024, 44(1): 251-258. |
| [3] | 董江湖, 宣良明, 王池, 赵晨熙, 王海峰, 严琼姣, 汪伟, 陈芬儿. 无过渡金属或无光催化剂条件下可见光促进喹喔啉酮C(3)—H官能团化研究进展[J]. 有机化学, 2024, 44(1): 111-136. |
| [4] | 贝文峰, 潘健, 冉冬梅, 刘伊琳, 杨震, 冯若昆. 基于钴催化吲哚酰胺与二炔和单炔的[4+2]环化反应合成γ-咔啉酮[J]. 有机化学, 2023, 43(9): 3226-3238. |
| [5] | 周然, 袁春梅, 张桃, 毛飘, 刘燚, 孟开妮, 幸惠, 薛伟. 含喹唑啉酮的查尔酮衍生物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(9): 3196-3209. |
| [6] | 唐菁, 罗文坤, 周俊. 氮杂螺[4.5]三烯酮衍生物的合成研究进展[J]. 有机化学, 2023, 43(9): 3006-3034. |
| [7] | 丁卫忠, 张炳文, 薛彦青, 林雨琦, 汤志军, 王婧, 杨文超, 王晓峰, 刘文. 禾谷镰刀菌中一个新的聚酮类化合物[J]. 有机化学, 2023, 43(9): 3319-3322. |
| [8] | 刘长俊, 胡慧玲, 刘宬宏, 朱超杰, 唐天地. 介孔ETS-10沸石担载Pd高效催化内炔氧化制备1,2-二酮[J]. 有机化学, 2023, 43(8): 2953-2960. |
| [9] | 吴文倩, 陈春霞, 彭进松, 李占宇. 羰基α-位胺化反应研究进展[J]. 有机化学, 2023, 43(8): 2743-2763. |
| [10] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [11] | 雷容超, 兰文捷, 李梦竹, 傅滨. 苯并磺内酰胺联吡唑化合物的简便合成[J]. 有机化学, 2023, 43(7): 2553-2560. |
| [12] | 许晓萍, 张翼飞, 莫小渝, 江俊. 铑催化3-重氮吲哚-2-亚胺与吡唑啉酮的C—H官能团化反应制备3-吡唑基吲哚[J]. 有机化学, 2023, 43(7): 2519-2527. |
| [13] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [14] | 秦娇, 陈杰, 苏艳. 无过渡金属催化的α-溴代茚酮自由基裂解反应合成(2-氰基苯基)乙酸-2,2,6,6-四甲基哌啶酯[J]. 有机化学, 2023, 43(6): 2171-2177. |
| [15] | 梁陆祺, 奚娟, 姜若楠, 杨艺, 孙丰钢, 张立志, 李新进, 刘会. 镍催化硫酯转移反应合成芳基硫酯[J]. 有机化学, 2023, 43(4): 1566-1573. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||