有机化学 ›› 2023, Vol. 43 ›› Issue (11): 3844-3860.DOI: 10.6023/cjoc202305013 上一篇 下一篇
综述与进展
侯学会a, 李议慧b, 张庆玲b, 刘俊桃a, 陈亚静b,*( )
)
收稿日期:2023-05-12
修回日期:2023-06-14
发布日期:2023-06-26
基金资助:
Xuehui Houa, Yihui Lib, Qingling Zhangb, Juntao Liua, Yajing Chenb( )
)
Received:2023-05-12
Revised:2023-06-14
Published:2023-06-26
Contact:
E-mail: Supported by:文章分享

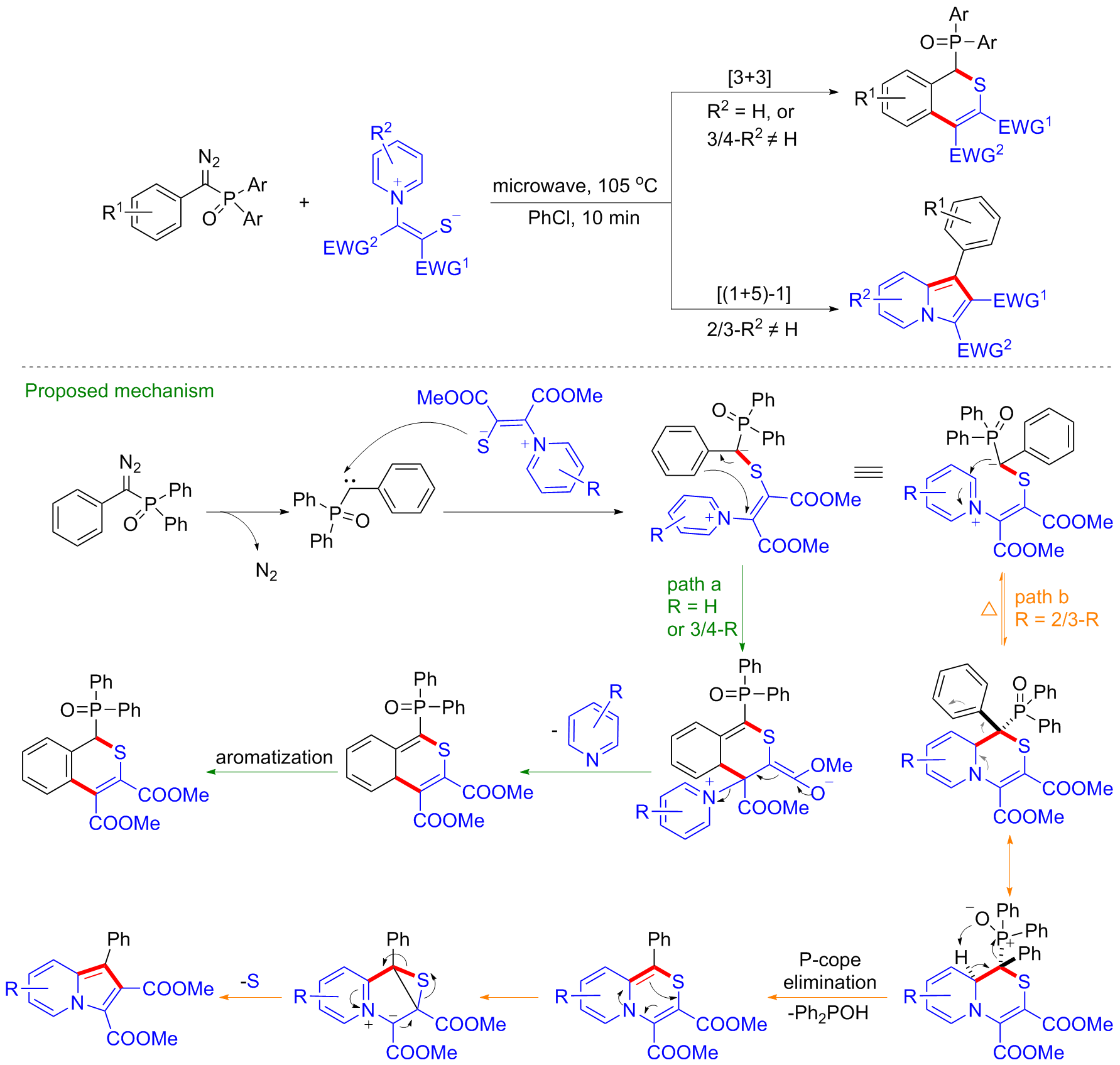
1,4-吡啶硫内鎓盐是一类重要的氮硫杂合成子, 其中的吡啶片段既可以作为离去基团, 也可以作为亲电反应位点参与反应, 构建多种类型的氮硫杂骨架. 因此, 近年来1,4-吡啶硫内鎓盐在含氮、含硫杂环化合物的合成应用中受到了极大的关注. 基于此, 系统综述了1,4-吡啶硫内鎓盐在两种反应模式([3+m]和[5+m]环合反应)下构建氮硫杂环的研究进展, 总结了其在环合反应及杂环化学中的应用, 并对该领域的研究前景进行了展望.
侯学会, 李议慧, 张庆玲, 刘俊桃, 陈亚静. 1,4-吡啶硫内鎓盐在有机合成中的研究与应用[J]. 有机化学, 2023, 43(11): 3844-3860.
Xuehui Hou, Yihui Li, Qingling Zhang, Juntao Liu, Yajing Chen. Research and Application of Pyridinium 1,4-Zwitterionic Thiolates in Organic Synthesis[J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3844-3860.

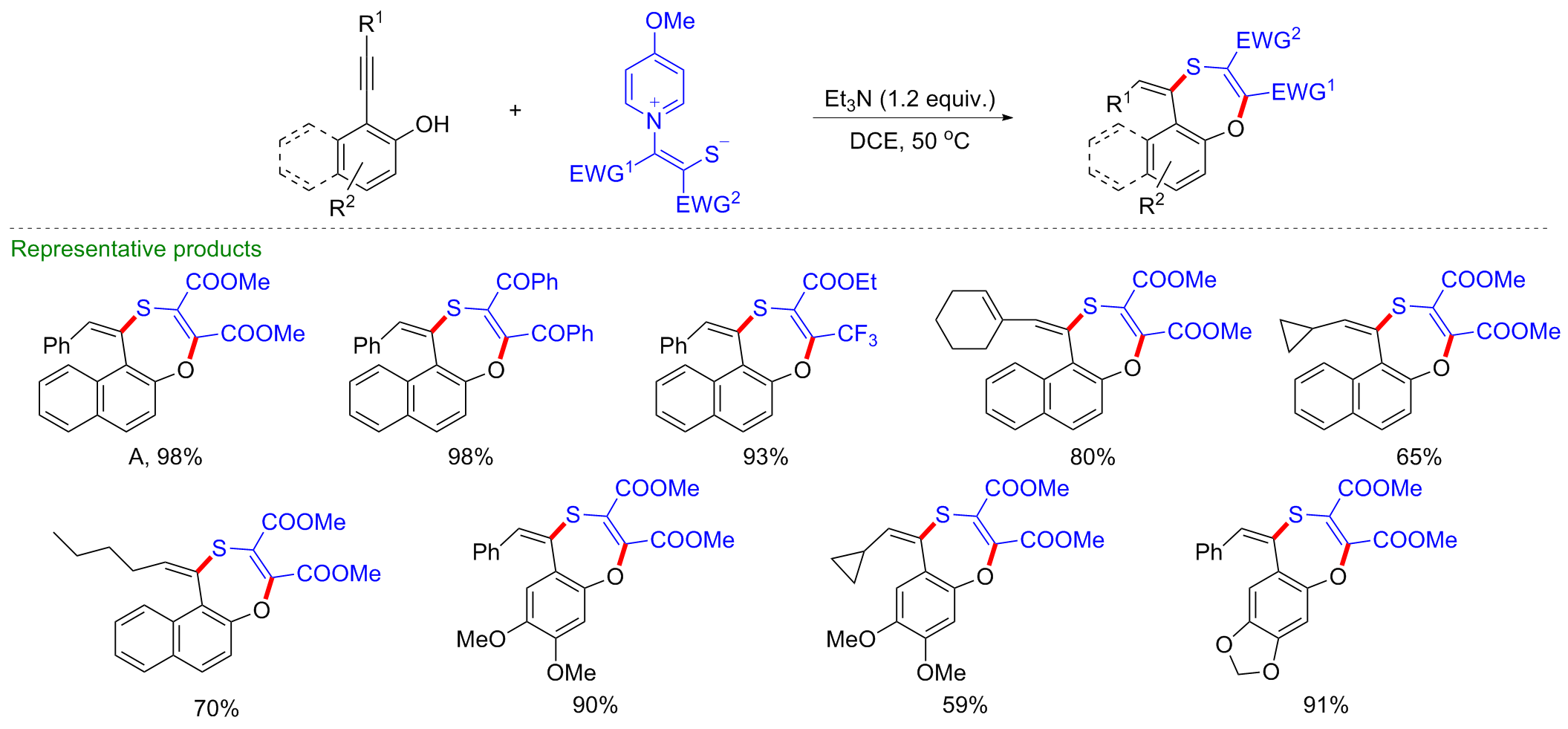

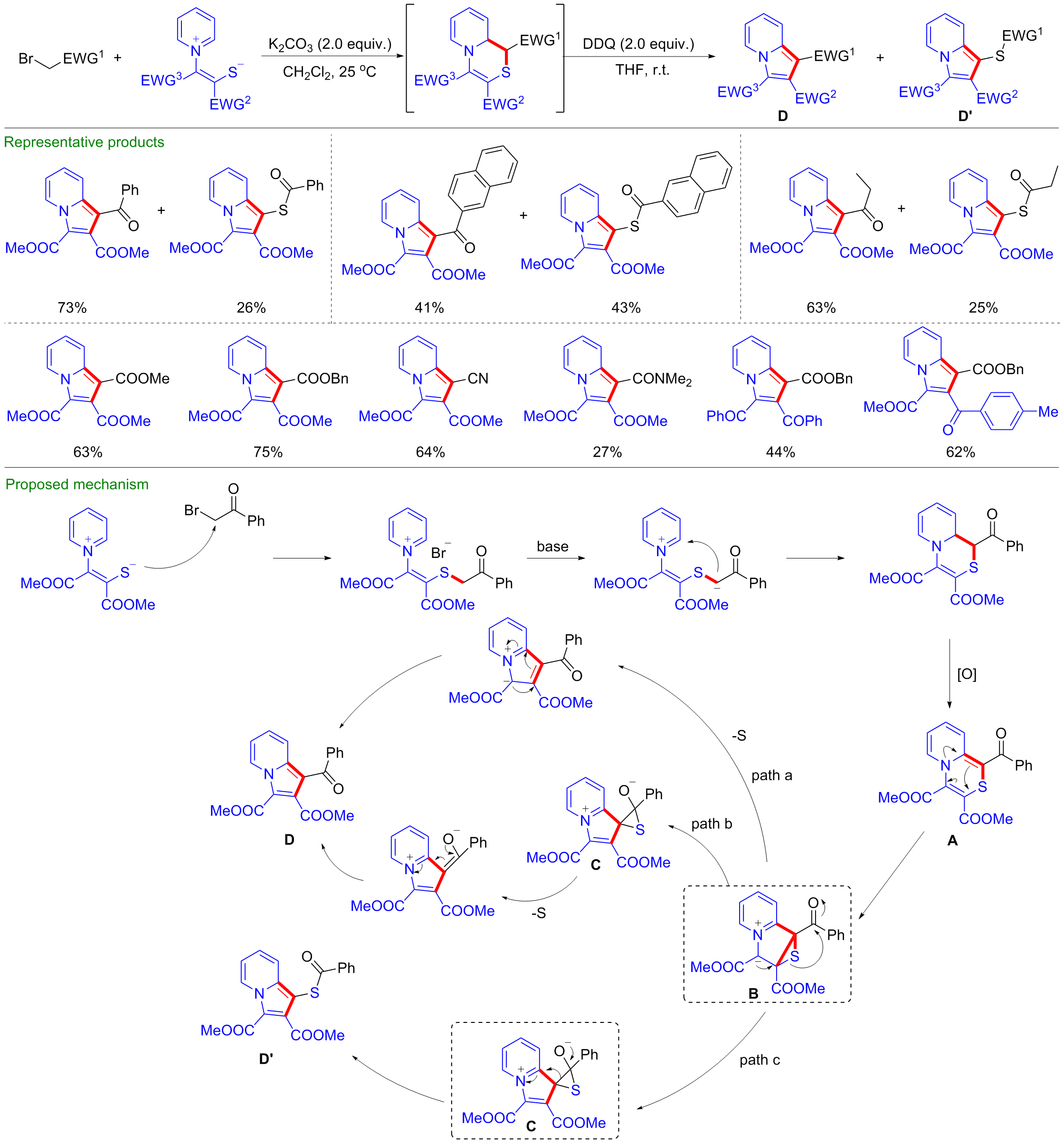
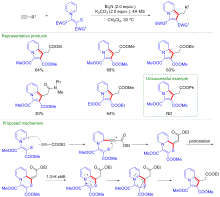

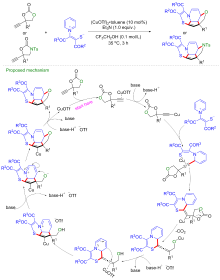
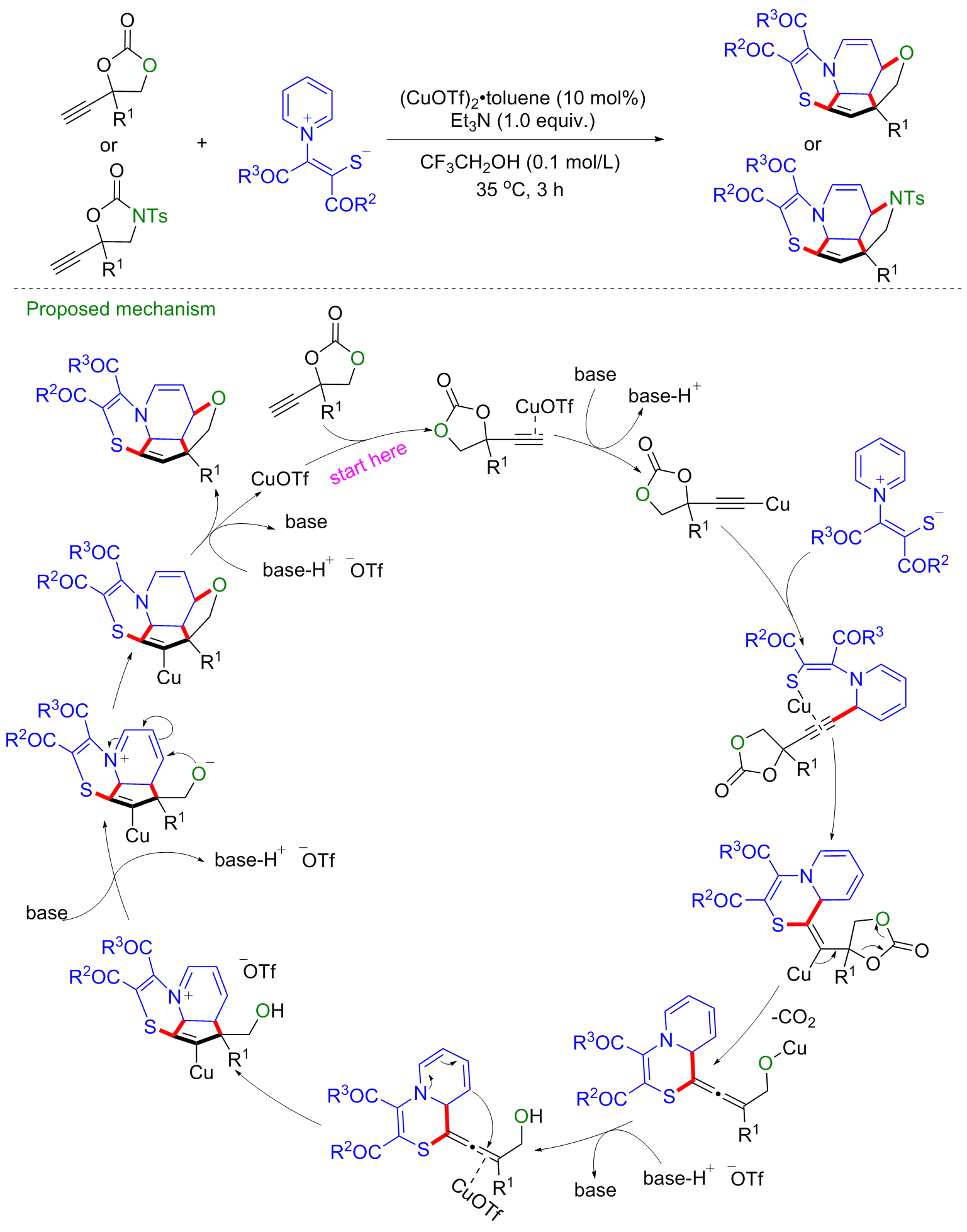


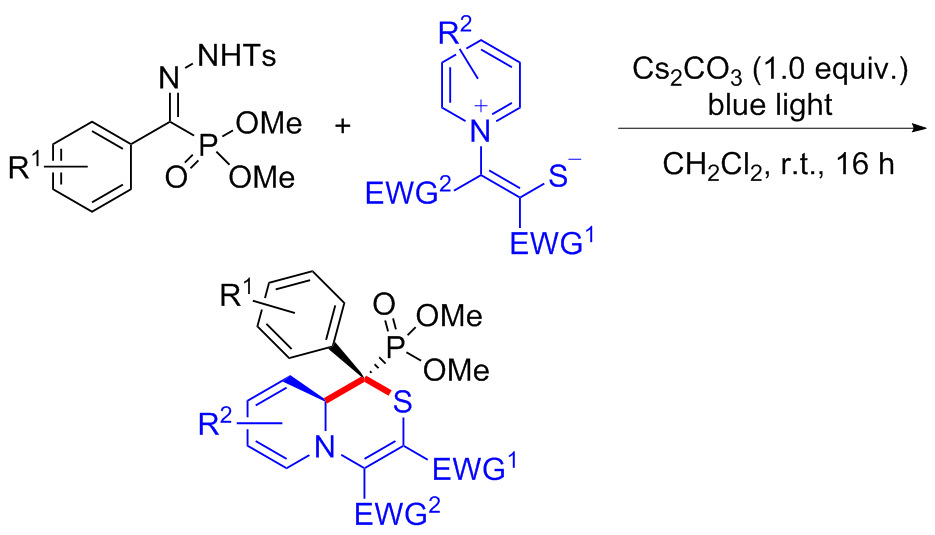
| [1] |
Vitaku, E.; Smith, D. T.; Njardarson, J. T. J. Med. Chem. 2014, 57, 10257.
doi: 10.1021/jm501100b pmid: 25255204 |
| [2] |
Zhang, J.; Zhang, C.; Zheng, Z.; Zhou, P.; Liu, W. Chin. J. Org. Chem. 2022, 42, 2745. (in Chinese)
doi: 10.6023/cjoc202204002 |
|
(张建涛, 张聪, 郑梓栋, 周鹏, 刘卫兵, 有机化学, 2022, 42, 2745.)
doi: 10.6023/cjoc202204002 |
|
| [3] |
Bhorali, P.; Sultana, S.; Gogoi, S. Asian J. Org. Chem. 2022, 11, e202100754.
doi: 10.1002/ajoc.v11.4 |
| [4] |
Sundaravelu, N.; Sangeetha, S.; Sekar, G. Org. Biomol. Chem. 2021, 19, 1459.
doi: 10.1039/d0ob02320e pmid: 33528480 |
| [5] |
Xiao, L.; Liu, G.; Ren, P.; Wu, T.; Lu, Y.; Kong, J. Chin. J. Org. Chem. 2022, 42, 1002. (in Chinese)
doi: 10.6023/cjoc202109038 |
|
(肖立伟, 刘光灿, 任萍, 吴彤桐, 卢玉伟, 孔洁, 有机化学, 2022, 42, 1002.)
doi: 10.6023/cjoc202109038 |
|
| [6] |
Moafi, L.; Ahadi, S.; Khavasi, H. R.; Bazgir, A. Synthesis 2011, 1399.
|
| [7] |
Cheng, B.; Wang, T. In Encyclopedia of Reagents for Organic Synthesis, Wiley-Interscience, Hoboken, 2021, pp. 1-4.
|
| [8] |
Wang, Z.-H.; You, Y.; Zhao, J.-Q.; Zhang, Y.-P.; Yin, J.-Q.; Yuan, W.-C. Molecules 2023, 28, 3059.
doi: 10.3390/molecules28073059 |
| [9] |
Huang, J.; Zhang, L.; Meng, X. Org. Chem. Front. 2023, 10, 2813.
doi: 10.1039/D3QO00228D |
| [10] |
Cheng, B.; Li, Y.; Wang, T.; Zhang, X.; Li, H.; Li, Y.; Zhai, H. Chem. Commun. 2019, 55, 14606.
doi: 10.1039/C9CC08326J |
| [11] |
Zhai, S.; Zhang, X.; Cheng, B.; Li, H.; Li, Y.; He, Y.; Wang, T.; Zhai, H. Chem. Commun. 2020, 56, 3085.
doi: 10.1039/D0CC00262C |
| [12] |
Cheng, B.; Duan, X.; Li, Y.; Zhang, X.; Li, H.; Wu, F.; Li, Y.; Wang, T.; Zhai, H. Eur. J. Org. Chem. 2020, 1896.
|
| [13] |
Wang, T.; Zhu, X.; Tao, Q.; Xu, W.; Sun, H.; Wu, P.; Cheng, B.; Zhai, H. Chin. Chem. Lett. 2021, 32, 3972.
doi: 10.1016/j.cclet.2021.04.021 |
| [14] |
Cheng, B.; Bao, B.; Xu, W.; Li, Y.; Li, H.; Zhang, X.; Li, Y.; Wang, T.; Zhai, H. Org. Biomol. Chem. 2020, 18, 2949.
doi: 10.1039/d0ob00224k pmid: 32242607 |
| [15] |
Yang, B.; Gao, S. Chem. Soc. Rev. 2018, 47, 7926.
doi: 10.1039/C8CS00274F |
| [16] |
Liao, H.-H.; Minoza, S.; Lee, S.-C.; Rueping, M. Chem.-Eur. J. 2022, 28, e202201112.
doi: 10.1002/chem.v28.46 |
| [17] |
Happy, S.; Junaid, M.; Yadagiri, D. Chem. Commun. 2023, 59, 29.
doi: 10.1039/D2CC05623B |
| [18] |
Wang, C.-C.; Liu, X.-H.; Wang, X.-L.; Cui, H.-P.; Ma, Z.-W.; Ding, D.; Liu, J.-T.; Meng, L. Chen, Y.-J. Adv. Synth. Catal. 2022, 364, 296.
doi: 10.1002/adsc.v364.2 |
| [19] |
Zhang, L.; Fang, L.; Huang, H.; Wang, C.; Gao, F.; Wang, Z. J. Org. Chem. 2021, 86, 18156.
doi: 10.1021/acs.joc.1c02433 |
| [20] |
He, Y.; Wu, P.; Zhang, X.; Wang, T.; Tao, Q.; Tao, Q.; Zhou, K.; Ouyang, Z.; Zhai, H.; Cheng, D.-J.; Cheng, B. Org. Chem. Front. 2022, 9, 4612.
doi: 10.1039/D2QO00735E |
| [21] |
Wang, C.-C.; Yang, Y.-T.; Wang, Q.-L.; Liu, X.-H.; Chen, Y.-J. Org. Chem. Front. 2022, 9, 4271.
doi: 10.1039/D2QO00612J |
| [22] |
Yao, Y.; Lin, B.; Wu, M.; Zhang, Y.; Huang, Y.; Han, X.; Han, X.; Weng, Z. Org. Biomol. Chem. 2022, 20, 8761.
doi: 10.1039/D2OB01749K |
| [23] |
Cheng, B.; Li, Y.; Wang, T.; Zhang, X.; Li, H.; He, Y.; Li, Y.; Zhai, H. J. Org. Chem. 2020, 85, 6794.
doi: 10.1021/acs.joc.0c00374 pmid: 32329339 |
| [24] |
Cheng, B.; Zhang, X.; Li, Y.; Li, H.; He, Y.; Li, Y.; Wang, T.; Zhai, H. Chem. Commun. 2020, 56, 8396.
doi: 10.1039/D0CC03446K |
| [25] |
Singh, G. S.; Mmatli, E. E. Eur. J. Med. Chem. 2011, 46, 5237.
doi: 10.1016/j.ejmech.2011.08.042 |
| [26] |
Sharma, V.; Kumar, V. Med. Chem. Res. 2014, 23, 3593.
doi: 10.1007/s00044-014-0940-1 |
| [27] |
Kim, E.; Lee, Y.; Lee, S.; Park, S. B. Acc. Chem. Res. 2015, 48, 538.
doi: 10.1021/ar500370v |
| [28] |
Cheng, B.; Li, H.; Duan, S.; Zhang, X.; He, Y.; Li, Y.; Wang, T.; Zhai, H. Org. Biomol. Chem. 2020, 18, 6253.
doi: 10.1039/d0ob01398f pmid: 32756728 |
| [29] |
Cheng, B.; Zhang, X.; Li, H.; He, Y.; Li, Y.; Sun, H.; Wang, T.; Zhai, H. Adv. Synth. Catal. 2020, 362, 4668.
doi: 10.1002/adsc.v362.21 |
| [30] |
Jin, Q.; Jiang, C.; Gao, M.; Zhang, D.; Hu, S.; Zhang, J. J. Org. Chem. 2021, 86, 15640.
doi: 10.1021/acs.joc.1c02175 |
| [31] |
Li, T.-T.; You, Y.; Sun, T.-J.; Zhang, Y.-P.; Zhao, J.-Q.; Wang, Z.-H.; Yuan, W.-C. Org. Lett. 2022, 24, 5120.
doi: 10.1021/acs.orglett.2c01959 |
| [32] |
Sun, S.; Wei, Y.; Xu, J. Org. Lett. 2022, 24, 6024.
doi: 10.1021/acs.orglett.2c02321 |
| [33] |
Wei, Y.; Sun, S.; Xu, J. Tetrahedron Lett. 2023, 120, 154444.
doi: 10.1016/j.tetlet.2023.154444 |
| [34] |
Monreal-Corona, R.; Díaz-Jiménez, À; Roglans, A.; Poster, A.; Pla-Quintana, A. Adv. Synth. Catal. 2023, 365, 760.
doi: 10.1002/adsc.v365.5 |
| [35] |
Li, W.; Wang, H.; Zhang, Y.; Zhao, L.; Guo, P.; Cao, H.; Liu, X. J. Org. Chem. 2023, 88, 7199.
doi: 10.1021/acs.joc.3c00447 |
| [36] |
Sun, S.; Wei, Y.; Xu, J. Org. Lett. 2023, 25, 2868.
doi: 10.1021/acs.orglett.3c00842 |
| [37] |
Cheng, B.; Li, Y.; Zhang, X.; Duan, S.; Li, H.; He, Y.; Li, Y.; Wang, T.; Zhai, H. Org. Lett. 2020, 22, 5817.
doi: 10.1021/acs.orglett.0c01888 pmid: 32648762 |
| [38] |
Duan, S.; Chen, C.; Chen, Y.; Jie, Y.; Luo, H.; Xu, Z.-F.; Cheng, B.; Li, C.-Y. Org. Chem. Front. 2021, 8, 6962.
doi: 10.1039/D1QO01237A |
| [39] |
Sun, S.; Wei, Y.; Xu, J. Chem. Commun. 2023, 59, 239.
doi: 10.1039/D2CC05483C |
| [1] | 张建涛, 张聪, 郑梓栋, 周鹏, 刘卫兵. 亚砜叶立德参与构建五/六元氮杂环的反应研究进展[J]. 有机化学, 2022, 42(9): 2745-2759. |
| [2] | 肖立伟, 刘光仙, 任萍, 吴彤桐, 卢玉伟, 孔洁. 单质硫: 合成含硫杂环的优质硫源[J]. 有机化学, 2022, 42(4): 1002-1012. |
| [3] | 洪科苗, 黄晶晶, 姚铭瀚, 徐新芳. 氮宾/炔烃复分解串联反应研究进展[J]. 有机化学, 2022, 42(2): 344-352. |
| [4] | 赵晓伟, 夏紫琴, 张曼, 周能能. 自由基介导的串联环化反应构建七元含氮/氧杂环化合物[J]. 有机化学, 2022, 42(12): 3995-4023. |
| [5] | 周欣悦, 梁宗显, 王晓娜. 近年来炔酰胺参与的成环反应研究进展[J]. 有机化学, 2021, 41(4): 1288-1318. |
| [6] | 周娅琴, 赵志恒, 曾亮, 李鸣, 何永辉, 谷利军. 卤素盐参与下有机电合成含氮杂环化合物的研究进展[J]. 有机化学, 2021, 41(3): 1072-1080. |
| [7] | 闫强, 范荣, 刘斌斌, 苏帅松, 王勃, 姚团利, 谭嘉靖. 苯炔参与的去芳构化反应研究进展[J]. 有机化学, 2021, 41(2): 455-470. |
| [8] | 周淑蕊, 温凯歌, 曾兴平. 亚胺及其类似物的催化不对称炔基化反应新进展[J]. 有机化学, 2021, 41(2): 471-489. |
| [9] | 张建涛, 周鹏, 肖朵朵, 刘卫兵. 1,3,5-三嗪烷合成含氮杂环的反应研究进展[J]. 有机化学, 2021, 41(11): 4154-4166. |
| [10] | 程辉成, 郭鹏虎, 陈冰, 姚嘉伟, 马姣丽, 胡炜杰, 纪红兵. 二苯并噻吩的合成研究进展[J]. 有机化学, 2021, 41(1): 94-104. |
| [11] | 张杰, 刘平, 孙培培. 自由基加成环化合成含氧或含氮杂环化合物的研究进展[J]. 有机化学, 2021, 41(1): 185-205. |
| [12] | 郑雨, 谢珍珍, 陈凯, 向皞月, 阳华. 有机催化在不对称构建含氮杂环化合物中的应用[J]. 有机化学, 2021, 41(1): 1-11. |
| [13] | 王旭才, 陈明, 张威, 张耀都, 任智卉, 关正辉. 钯催化β,γ-不饱和腙的5-exo-trig型氢酰胺化反应合成二氢吡唑[J]. 有机化学, 2020, 40(6): 1618-1624. |
| [14] | 马奔, 王刚刚, 周红艳, 杨靖亚. 碱金属盐催化1,2,4-三唑与α,β-不饱和酮及二酰亚胺的氮杂Michael反应[J]. 有机化学, 2020, 40(1): 115-124. |
| [15] | 王钰莹, 刘莉, 王也铭. 基于烯基叠氮合成含氮杂环化合物的研究进展[J]. 有机化学, 2018, 38(5): 1009-1028. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||