有机化学 ›› 2025, Vol. 45 ›› Issue (1): 276-285.DOI: 10.6023/cjoc202405010 上一篇 下一篇
研究论文
路星星a, 林誉凡a, 徐欢a, 张晓鸣a,*( ), 李雪生b, 凌云a, 杨新玲a,*(
), 李雪生b, 凌云a, 杨新玲a,*( )
)
收稿日期:2024-06-21
修回日期:2024-07-24
发布日期:2024-08-16
基金资助:
Xingxing Lua, Yufan Lina, Huan Xua, Xiaoming Zhanga( ), Xuesheng Lib, Yun Linga, Xinling Yanga(
), Xuesheng Lib, Yun Linga, Xinling Yanga( )
)
Received:2024-06-21
Revised:2024-07-24
Published:2024-08-16
Contact:
*E-mail: Supported by:文章分享

吡啶片段在农药设计中扮演着重要角色. 氟虫啶胺(flupyrimin, FLP)是一个含双吡啶结构的新型新烟碱类杀虫剂, 前期对FLP的结构改造发现, 含双吡啶结构的化合物N-[(6-氯吡啶-3-基)甲基]-3-甲基-N-(吡啶-2-基)苯磺酰胺(3c)具有一定的杀虫活性. 以化合物3c为先导, 设计合成了一系列含双吡啶结构的衍生物, 并进行生物活性评估. 测试结果发现目标化合物展现出一定的杀虫和抑菌活性, 其中N-[(6-氯吡啶-3-基)甲基]-N-甲基吡啶-2-胺(T1)和N-((6-氯吡啶- 3-基)甲基)-N-乙基吡啶-2-胺(T2)对大豆蚜(Aphis glycines)表现出较好的杀虫活性, LC50值分别为11.04和25.12 mg/L; N-[(6-氯吡啶-3-基)甲基]-N-戊基吡啶-2-胺(T5)和N-[(6-氯吡啶-3-基)甲基]-N-己基吡啶-2-胺(T6)对番茄灰霉病菌(Botrytis cinerea)表现出较好的抑菌活性, EC50值分别为7.45和10.54 mg/L. 蜜蜂毒性测试结果表明, 化合物T1对意大利蜜蜂(Apis mellifera)安全. 此外通过分子动力学模拟对化合物的杀虫机制研究发现, 化合物T1维持了与AChBP关键氨基酸的水桥作用, 结合自由能优于先导化合物3c. 本研究对探索吡啶类化合物的农用生物活性具有重要的指导意义.
路星星, 林誉凡, 徐欢, 张晓鸣, 李雪生, 凌云, 杨新玲. 新型双吡啶类化合物的设计、合成及生物活性研究[J]. 有机化学, 2025, 45(1): 276-285.
Xingxing Lu, Yufan Lin, Huan Xu, Xiaoming Zhang, Xuesheng Li, Yun Ling, Xinling Yang. Design, Synthesis and Bioactivity Research of Novel Bipyridine Compounds[J]. Chinese Journal of Organic Chemistry, 2025, 45(1): 276-285.
| Compd. | 200 mg•L-1 | 100 mg•L-1 |
|---|---|---|
| T1 | 100 | 80.96±5.42 |
| T2 | 95.70±2.72 | 75.48±5.53 |
| T3 | 71.32±6.77 | |
| T4 | 82.80±5.93 | 12.77±2.41 |
| T5 | 59.14±7.58 | |
| T6 | 81.91±4.10 | 2.06±2.39 |
| T7 | 78.49±7.20 | |
| T8 | 95.80±1.33 | 4.62±0.66 |
| T9 | 62.72±10.22 | |
| T10 | 95.70±2.72 | 23.91±0.68 |
| T11 | 19.61±7.58 | |
| T12 | 3.92±3.60 | |
| T13 | 46.43±1.83 | 37.84±2.19 |
| T14 | 79.70±2.24 | |
| T15 | 33.64±6.22 | |
| T16 | 70.85±2.34 | |
| 3c | 72.09±3.14 | 41.67±6.80 |
| FLP | 100 | 100 |
| Pymetrozine | 86.64±7.05 | 76.13±4.80 |
| Compd. | 200 mg•L-1 | 100 mg•L-1 |
|---|---|---|
| T1 | 100 | 80.96±5.42 |
| T2 | 95.70±2.72 | 75.48±5.53 |
| T3 | 71.32±6.77 | |
| T4 | 82.80±5.93 | 12.77±2.41 |
| T5 | 59.14±7.58 | |
| T6 | 81.91±4.10 | 2.06±2.39 |
| T7 | 78.49±7.20 | |
| T8 | 95.80±1.33 | 4.62±0.66 |
| T9 | 62.72±10.22 | |
| T10 | 95.70±2.72 | 23.91±0.68 |
| T11 | 19.61±7.58 | |
| T12 | 3.92±3.60 | |
| T13 | 46.43±1.83 | 37.84±2.19 |
| T14 | 79.70±2.24 | |
| T15 | 33.64±6.22 | |
| T16 | 70.85±2.34 | |
| 3c | 72.09±3.14 | 41.67±6.80 |
| FLP | 100 | 100 |
| Pymetrozine | 86.64±7.05 | 76.13±4.80 |
| Compd. | y=ax+b | LC50/ (mg•L-1) | 95% confiden- ce interval | r |
|---|---|---|---|---|
| T1 | y=1.17x-1.23 | 11.04 | 6.38~15.65 | 0.98 |
| T2 | y=0.74x-1.00 | 25.12 | 16.97~35.77 | 0.99 |
| 3c | y=1.85x-3.71 | 102.38 | 87.35~123.14 | 0.97 |
| Pymetrozine | y=0.70x-0.54 | 5.85 | 2.51~9.78 | 0.98 |
| FLP | y=0.84x+0.61 | 0.20 | 0.12~0.29 | 0.98 |
| Compd. | y=ax+b | LC50/ (mg•L-1) | 95% confiden- ce interval | r |
|---|---|---|---|---|
| T1 | y=1.17x-1.23 | 11.04 | 6.38~15.65 | 0.98 |
| T2 | y=0.74x-1.00 | 25.12 | 16.97~35.77 | 0.99 |
| 3c | y=1.85x-3.71 | 102.38 | 87.35~123.14 | 0.97 |
| Pymetrozine | y=0.70x-0.54 | 5.85 | 2.51~9.78 | 0.98 |
| FLP | y=0.84x+0.61 | 0.20 | 0.12~0.29 | 0.98 |
| Compd. | Pythium aphanidermatum | Magnaporthe oryzae | Valsa mali | Botrytis cinerea | Phytophthora capsici |
|---|---|---|---|---|---|
| T1 | 7.42±3.24 | 19.62±1.48 | 8.42±0.74 | 45.65±2.91 | 8.03±0 |
| T2 | 16.79±0.65 | 25.77±0.33 | 6.57±1.32 | 47.39±1.43 | 14.72±0.84 |
| T3 | 49.03±0.75 | 31.15±0.33 | 2.88±0.61 | 44.35±0.87 | 32.69±0.36 |
| T4 | 77.51±1.84 | 43.85±1.92 | 9.53±1.68 | 93.48±0.38 | 45.65±1.25 |
| T5 | 52.77±4.90 | 70.38±4.19 | 16.54±1.11 | 100 | 34.78±0.84 |
| T6 | 11.54±1.91 | 64.23±2.39 | 38.70±1.11 | 91.30±0 | 14.30±0.36 |
| T7 | 63.27±2.02 | 27.69±0.54 | 9.16±1.11 | 40.87±0.61 | 41.47±0 |
| T8 | 63.27±0.65 | 32.69±3.28 | 15.07±1.85 | 40.00±1.44 | 49.83±0 |
| T9 | 35.53±1.50 | 31.92±0.64 | 26.14±5.17 | 47.83±2.13 | 50.25±0.36 |
| T10 | 7.05±1.59 | 45.38±1.15 | 49.04±0.64 | 60.00±0.61 | 44.40±0.36 |
| T11 | 3.30±0.65 | 23.85±0.38 | 22.82±0.32 | 38.70±5.08 | 16.81±0.36 |
| T12 | 5.55±1.91 | 36.15±1.28 | 30.21±1.09 | 50.00±0.38 | 23.49±0.69 |
| T13 | 40.78±2.73 | 40.00±1.44 | 32.42±0.61 | 46.09±0.87 | 31.86±0.36 |
| T14 | 38.91±5.26 | 20.38±1.00 | 14.33±0 | 46.09±0.87 | 17.22±1.25 |
| T15 | 25.04±5.00 | 31.15±3.66 | 6.20±1.11 | 40.00±0.75 | 18.06±0.59 |
| T16 | 40.40±3.53 | 78.46±3.88 | 36.85±2.05 | 46.96±0.75 | 33.95±0.42 |
| 3c | 13.97±0.83 | 54.02±0.50 | 52.86±0.65 | 47.66±0.75 | 25.30±0.75 |
| Pyraclostrobin | 100 | 100 | 100 | 24.35±1.30 | 4.68±0 |
| Prothioconazole | 57.77±1.48 | 100 | 100 | 100 | 66.76±1.12 |
| Compd. | Pythium aphanidermatum | Magnaporthe oryzae | Valsa mali | Botrytis cinerea | Phytophthora capsici |
|---|---|---|---|---|---|
| T1 | 7.42±3.24 | 19.62±1.48 | 8.42±0.74 | 45.65±2.91 | 8.03±0 |
| T2 | 16.79±0.65 | 25.77±0.33 | 6.57±1.32 | 47.39±1.43 | 14.72±0.84 |
| T3 | 49.03±0.75 | 31.15±0.33 | 2.88±0.61 | 44.35±0.87 | 32.69±0.36 |
| T4 | 77.51±1.84 | 43.85±1.92 | 9.53±1.68 | 93.48±0.38 | 45.65±1.25 |
| T5 | 52.77±4.90 | 70.38±4.19 | 16.54±1.11 | 100 | 34.78±0.84 |
| T6 | 11.54±1.91 | 64.23±2.39 | 38.70±1.11 | 91.30±0 | 14.30±0.36 |
| T7 | 63.27±2.02 | 27.69±0.54 | 9.16±1.11 | 40.87±0.61 | 41.47±0 |
| T8 | 63.27±0.65 | 32.69±3.28 | 15.07±1.85 | 40.00±1.44 | 49.83±0 |
| T9 | 35.53±1.50 | 31.92±0.64 | 26.14±5.17 | 47.83±2.13 | 50.25±0.36 |
| T10 | 7.05±1.59 | 45.38±1.15 | 49.04±0.64 | 60.00±0.61 | 44.40±0.36 |
| T11 | 3.30±0.65 | 23.85±0.38 | 22.82±0.32 | 38.70±5.08 | 16.81±0.36 |
| T12 | 5.55±1.91 | 36.15±1.28 | 30.21±1.09 | 50.00±0.38 | 23.49±0.69 |
| T13 | 40.78±2.73 | 40.00±1.44 | 32.42±0.61 | 46.09±0.87 | 31.86±0.36 |
| T14 | 38.91±5.26 | 20.38±1.00 | 14.33±0 | 46.09±0.87 | 17.22±1.25 |
| T15 | 25.04±5.00 | 31.15±3.66 | 6.20±1.11 | 40.00±0.75 | 18.06±0.59 |
| T16 | 40.40±3.53 | 78.46±3.88 | 36.85±2.05 | 46.96±0.75 | 33.95±0.42 |
| 3c | 13.97±0.83 | 54.02±0.50 | 52.86±0.65 | 47.66±0.75 | 25.30±0.75 |
| Pyraclostrobin | 100 | 100 | 100 | 24.35±1.30 | 4.68±0 |
| Prothioconazole | 57.77±1.48 | 100 | 100 | 100 | 66.76±1.12 |
| Compd. | y=ax+b | EC50/ (mg•L-1) | 95% confiden- ce interval | r |
|---|---|---|---|---|
| T4 | y=2.15x-2.60 | 17.50 | 12.07~27.93 | 0.95 |
| T5 | y=2.22x-1.93 | 7.45 | 5.79~9.53 | 0.98 |
| T6 | y=1.77x-1.78 | 10.54 | 9.08~12.29 | 0.98 |
| Prothioconazole | y=0.85x-0.59 | 4.90 | 3.52~7.09 | 0.99 |
| Compd. | y=ax+b | EC50/ (mg•L-1) | 95% confiden- ce interval | r |
|---|---|---|---|---|
| T4 | y=2.15x-2.60 | 17.50 | 12.07~27.93 | 0.95 |
| T5 | y=2.22x-1.93 | 7.45 | 5.79~9.53 | 0.98 |
| T6 | y=1.77x-1.78 | 10.54 | 9.08~12.29 | 0.98 |
| Prothioconazole | y=0.85x-0.59 | 4.90 | 3.52~7.09 | 0.99 |
| Compd. | Mutagenisis | Carcinogenicity | Bee toxicity | Acute oral toxicity in rats | |
|---|---|---|---|---|---|
| LD50/(mg•kg-1) | Toxicity grade (US EPA)a | ||||
| T1 | None | None | Low | 637.85 | Low |
| T2 | None | None | Low | 522.07 | Low |
| T3 | None | None | Low | 436.35 | Low |
| T4 | None | None | Low | 451.86 | Low |
| T5 | None | None | Low | 527.16 | Low |
| T6 | None | None | Low | 712.76 | Low |
| T7 | None | None | Low | 752.48 | Low |
| T8 | None | None | Low | —b | |
| T9 | None | None | Low | 555.54 | Low |
| T10 | None | None | Low | 357.33 | Moderate |
| T11 | None | None | Low | 1087.59 | Low |
| T12 | None | None | Low | 993.15 | Low |
| T13 | None | None | Low | 1067.69 | Low |
| T14 | Low | High | Low | 564.57 | Low |
| T15 | None | None | Low | 806.12 | Low |
| T16 | None | None | Low | 2454.79 | Low |
| Compd. | Mutagenisis | Carcinogenicity | Bee toxicity | Acute oral toxicity in rats | |
|---|---|---|---|---|---|
| LD50/(mg•kg-1) | Toxicity grade (US EPA)a | ||||
| T1 | None | None | Low | 637.85 | Low |
| T2 | None | None | Low | 522.07 | Low |
| T3 | None | None | Low | 436.35 | Low |
| T4 | None | None | Low | 451.86 | Low |
| T5 | None | None | Low | 527.16 | Low |
| T6 | None | None | Low | 712.76 | Low |
| T7 | None | None | Low | 752.48 | Low |
| T8 | None | None | Low | —b | |
| T9 | None | None | Low | 555.54 | Low |
| T10 | None | None | Low | 357.33 | Moderate |
| T11 | None | None | Low | 1087.59 | Low |
| T12 | None | None | Low | 993.15 | Low |
| T13 | None | None | Low | 1067.69 | Low |
| T14 | Low | High | Low | 564.57 | Low |
| T15 | None | None | Low | 806.12 | Low |
| T16 | None | None | Low | 2454.79 | Low |
| Compd. | Mortality rate/% | Bee toxicitya | |
|---|---|---|---|
| Oral toxicity | Contact toxicity | ||
| Blank control | 10.00 | 10.00 | |
| Solvent control | 3.33 | 6.67 | |
| T1b | 0.00 | 3.33 | Low |
| Pymetrozine | LD50>60 μg/bee | LD50>12 μg/bee | Low |
| Imidacloprid | LD50=2.82×10-2 μg/bee | LD50=5.78×10-2 μg/bee | High |
| Compd. | Mortality rate/% | Bee toxicitya | |
|---|---|---|---|
| Oral toxicity | Contact toxicity | ||
| Blank control | 10.00 | 10.00 | |
| Solvent control | 3.33 | 6.67 | |
| T1b | 0.00 | 3.33 | Low |
| Pymetrozine | LD50>60 μg/bee | LD50>12 μg/bee | Low |
| Imidacloprid | LD50=2.82×10-2 μg/bee | LD50=5.78×10-2 μg/bee | High |
| Ligand | ΔΕvdw/(kcal•mol-1) | ΔEele/(kcal•mol-1) | ΔGgas/(kcal•mol-1) | ΔGsol/(kcal•mol-1) | ΔGbind/(kcal•mol-1) |
|---|---|---|---|---|---|
| T1 | -31.03±2.33 | -14.50±1.36 | -46.53±2.94 | 22.05±1.10 | -24.48±2.90 |
| 3c | -38.12±2.08 | -8.41±8.22 | -46.52±9.16 | 22.83±5.08 | -23.69±4.58 |
| Ligand | ΔΕvdw/(kcal•mol-1) | ΔEele/(kcal•mol-1) | ΔGgas/(kcal•mol-1) | ΔGsol/(kcal•mol-1) | ΔGbind/(kcal•mol-1) |
|---|---|---|---|---|---|
| T1 | -31.03±2.33 | -14.50±1.36 | -46.53±2.94 | 22.05±1.10 | -24.48±2.90 |
| 3c | -38.12±2.08 | -8.41±8.22 | -46.52±9.16 | 22.83±5.08 | -23.69±4.58 |
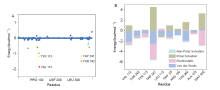
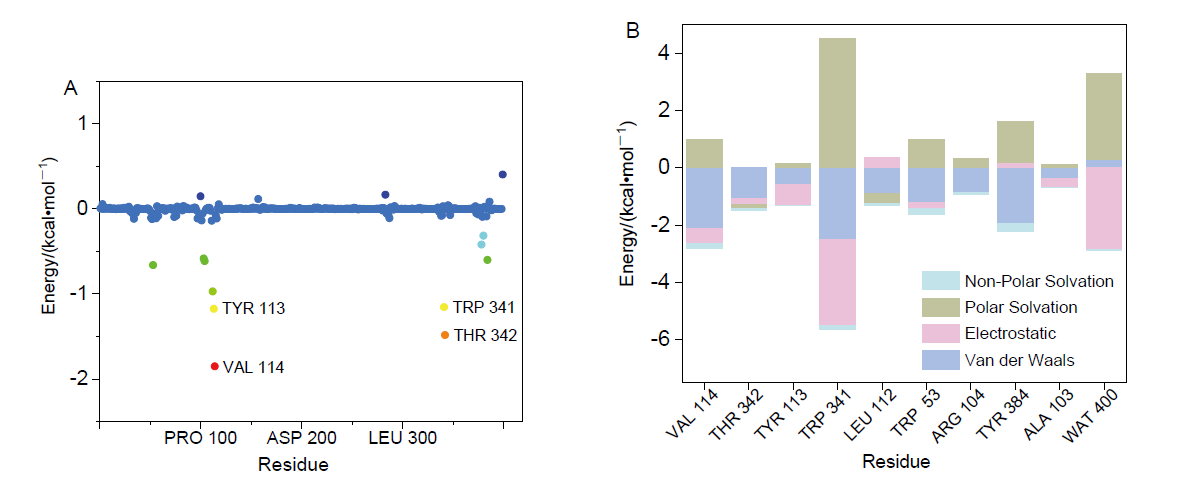
| [1] |
Li, D.-K.; Zheng, Q.-C.; Li, Z.-Y.; Xie, Y.; Zhang, L.-X.; Zhao, L.-K.; Yang, Y.; Liu, J.; Na, R.-S. Agrochemicals 2022, 61, 10 (in Chinese).
|
|
(李登奎, 郑乾成, 李志愿, 谢勇, 张立新, 赵利康, 杨莹, 刘佳, 那日松, 农药, 2022, 61, 10.)
|
|
| [2] |
Tsukamoto, M.; Nakamura, T.; Kimura, H.; Nakayama, H. J. Pestic. Sci. 2021, 46, 2.
|
| [3] |
DeBoer, G. J.; Thornburgh, S.; Gilbert, J.; Gast, R. E. Pest Manage. Sci. 2011, 67, 3.
|
| [4] |
Edmunds, A. J. F. Chimia 2022, 76, 7.
|
| [5] |
Epp, J. B.; Schmitzer, P. R.; Crouse, G. D. Pest Manage. Sci. 2018, 74, 1.
|
| [6] |
Li, Y.-W.; Tang, Y.-D.; Xue, Z.-W.; Wang, Y.; Shi, Y.-F.; Gao, X.-H.; Li, X.; Li, G.-X.; Li, F.; Lu, L.; Miao, J.-Q.; Liu, X.-L. J. Agric. Food Chem. 2023, 71, 4.
|
| [7] |
Babij, N. R.; Choy, N.; Cismesia, M. A.; Couling, D. J.; Hough, N. M.; Johnson, P. L.; Klosin, J.; Li, X.-Y.; Lu, Y.; McCusker, E. O.; Meyer, K. G.; Renga, J. M.; Rogers, R. B.; Stockman, K. E.; Webb, N. J.; Whiteker, G. T.; Zhu, Y.-M. Green Chem. 2020, 22, 18.
|
| [8] |
Jackson, K. L.; Yin, J.-F.; Csinos, A. S.; Ji, P.-S. Crop Prot. 2010, 29, 12.
|
| [9] |
Royals, B.; Brandenburg, R.; Hare, A.; Jordan, D.; Taylor, S.; Malone, S. Crop, Forage Turfgrass Manage. 2020, 6, 1.
|
| [10] |
Lahm, G.P.; Cordova, D.; Barry, J. D. Bioorg. Med. Chem. 2009, 17, 12.
|
| [11] |
Sheng, Z.-B.; Wang, J.; Pei, H.-Y.; Gao, Y.-X.; Zhang, J.; Zhang, L.-X. Agrochemicals 2021, 60, 1 (in Chinese).
|
|
(盛祝波, 汪杰, 裴鸿艳, 高一星, 张静, 张立新, 农药, 2021, 60, 1.)
|
|
| [12] |
Tan, H.-J. World Agrochem. 2019, 41, 5 (in Chinese).
|
|
(谭海军, 世界农药, 2019, 41, 5.)
|
|
| [13] |
Kleier, D.; Holden, I.; Casida, J. E.; Ruzo, L. O. J. Agric. Food Chem. 1985, 33, 5.
|
| [14] |
Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. J. Agric. Food Chem. 2011, 59, 7.
|
| [15] |
Casida, J. E.; Durkin, K. A. Annu. Rev. Entomol. 2013, 58, 99.
|
| [16] |
Nauen, R.; Jeschke, P.; Velten, R.; Beck, M. E.; Ebbinghaus- Kintscher, U.; Thielert, W.; Wölfel, K.; Haas, M.; Kunz, K.; Raupach, G. Pest Manage. Sci. 2015, 71, 6.
|
| [17] |
Onozaki, Y.; Horikoshi, R.; Ohno, I.; Kitsuda, S.; Durkin, K. A.; Suzuki, T.; Asahara, C.; Hiroki, N.; Komabashiri, R.; Shimizu, R.; Furutani, S.; Ihara, M.; Matsuda, K.; Mitomi, M.; Kagabu, S.; Uomoto, K.; Tomizawa, M. J. Agric. Food Chem. 2017, 65, 36.
|
| [18] |
Tan, H.-J. World Pestic. 2021, 43, 1 (in Chinese).
|
|
(谭海军, 世界农药, 2021, 43, 1.)
|
|
| [19] |
Lu, X.-X.; Xu, H.; Zhang, X.-M.; Sun, T.-D.; Lin, Y.-F.; Zhang, Y.-H.; Li, H.-H.; Li, X.-S.; Yang, X.-L.; Duan, H.-X.; Ling, Y. Molecules 2022, 27, 18.
|
| [20] |
Lu, X.-X.; Xu, H.; Zhang, X.-M.; Sun, T.-D.; Lin, Y.-F.; Li, H.-H.; Li, X.-S.; Zhang, L.; Duan, H.-X.; Yang, X.-L.; Ling, Y. J. Agric. Food Chem. 2023, 71, 49.
|
| [21] |
Yang, Z.-K.; Wu, X.; Zhang, J.-L.; Lu, X.-X.; Li, X.; Jiang, Z.-Y.; Song, D.-L.; Duan, H.-X.; Yang, X.-L. Chin. J. Org. Chem. 2021, 41, 7 (in Chinese).
|
|
(杨朝凯, 武霞, 张晋陆, 路星星, 李想, 蒋志洋, 宋敦伦, 段红霞, 杨新玲, 有机化学, 2021, 41, 7.)
|
|
| [22] |
Adriaanse, P.; Arce, A.; Focks, A.; Ingels, B.; Jölli, D.; Lambin, S.; Rundlöf, M.; Süßenbach, D.; Del Aguila, M.; Ercolano, V.; Ferilli, F.; Ippolito, A.; Szentes, C.; Neri, F. M.; Padovani, L.; Rortais, A.; Wassenberg, J.; Auteri, D. EFSA J. 2023, 21, 7989.
|
| [23] |
Bailey, J.; Scott-Dupree, C.; Harris, R.; Tolman, J.; Harris, B. Apidologie 2007, 36, 4.
|
| [24] |
Mommarets, V.; Sterk, G.; Smagghe, G. Pest Manage. Sci. 2006, 62, 8.
|
| [25] |
Oruc, H. H.; Hranitz, J. M.; Sorucu, A.; Duell, M.; Cakmak, I.; Aydin, L.; Orman, A. J. Econ. Entomol. 2012, 105, 6.
|
| [26] |
Talley, T. T.; Harel, M.; Hibbs, R. E.; Radić, Z.; Tomizawa, M.; Casida, J. E.; Taylor, P. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 21.
|
| [27] |
Elbaramawi, S. S.; Ibrahim, S. M.; Lashine, E. S. M.; El-Sadek, M. E.; Mantzourani, E.; Simons, C. J. Mol. Graphics Modell. 2017, 73, 36.
|
| [28] |
Case, D. A.; Cheatham, T. E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K. M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R. J. J. Comput. Chem. 2005, 26, 16.
|
| [29] |
Maier, J. A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K. E.; Simmerling, C. J. Chem. Theory Comput. 2015, 11, 8.
|
| [30] |
Wang, J.; Wolf, R. M.; Caldwell, J. W.; Kollman, P. A.; Case, D. A. J. Comput. Chem. 2004, 25, 9.
|
| [1] | 田海平, 刘东东, 裴鸿艳, 叶家麟, 郑子锐, 高一星, 李昌兴, 田欢, 张静, 张立新. 新型苯基吡唑类衍生物的设计、合成和杀虫活性研究[J]. 有机化学, 2025, 45(1): 227-239. |
| [2] | 刘吉永, 吴明慧, 相君成, 庞怀林, 李斌, 吕亮. 新型含(卤代)烷氧基类双酰胺化合物的合成、杀虫活性及构效关系研究[J]. 有机化学, 2024, 44(5): 1584-1591. |
| [3] | 赵薇, 李凤宇, 赵伟, 查润, 史彩华, 徐志红. 1,2,3-苯并三嗪-4-酮噁二唑硫醚(砜)甲基衍生物的设计、合成及杀菌和杀线虫活性[J]. 有机化学, 2024, 44(11): 3446-3455. |
| [4] | 霍海波, 李桂霞, 王世军, 韩春, 师宝君, 李健. 新型γ-咔啉衍生物的合成及其抑菌活性研究[J]. 有机化学, 2024, 44(1): 204-215. |
| [5] | 刘敏, 杨冬燕, 肖玉梅, 苏旺苍, 赵峰海, 覃兆海. 5-硝基亚氨基[1,4-2H]-1,2,4-三唑啉烯式吡虫啉类似物的合成及生物活性研究[J]. 有机化学, 2023, 43(8): 2790-2799. |
| [6] | 张紫婵, 孙洋, 华晟, 徐宝琳, 张敏, 赵勤, 郑丹丹, 王杨, 鞠剑峰, 石玉军, 戴红. 新型含异噁唑单元的吡唑酰胺类衍生物的合成及杀虫活性[J]. 有机化学, 2023, 43(4): 1435-1443. |
| [7] | 徐欢, 吴鸿飞, 张晓鸣, 路星星, 孙腾达, 亓悦, 林誉凡, 杨新玲, 张莉, 凌云. 含1,2,3,4-四氢异喹啉片段磺酰肼和酰肼类化合物的设计、合成及生物活性研究[J]. 有机化学, 2023, 43(2): 725-733. |
| [8] | 汪蕾, 于淑晶, 杨娜, 王宝雷. 新型含二氢喹唑啉酮的咖啡因衍生物的合成及生物活性研究[J]. 有机化学, 2023, 43(1): 299-307. |
| [9] | 李文娟, 张睿, 蔡志华, 韩小强, 何林, 代斌. 苯炔[3+2]环加成反应构建三氟甲基取代的苯并环状亚砜亚胺衍生物及其杀棉蚜活性研究[J]. 有机化学, 2022, 42(9): 2832-2839. |
| [10] | 王长凯, 孙腾达, 张学博, 杨新玲, 路星星, 徐欢, 石发胜, 张莉, 凌云. 新型含氟吡唑酰肼类化合物的设计合成与生物活性研究[J]. 有机化学, 2022, 42(5): 1527-1536. |
| [11] | 王秀, 段文贵, 林桂汕, 李宝谕, 张文静, 雷福厚. 含天然蒎烯结构的4-酰基-3-氨基-1,2,4-三唑-硫醚衍生物的合成、抑菌活性、三维定量构效关系及分子对接研究[J]. 有机化学, 2022, 42(3): 871-883. |
| [12] | 崔玉成, 陈美桦, 林桂汕, 段文贵, 李晴敏, 邹壬萱, 岑波. 含偕二甲基环丙烷结构的1,3,4-噻二唑-脲化合物的合成、抑菌活性及分子对接研究[J]. 有机化学, 2022, 42(11): 3784-3797. |
| [13] | 陈澍, 邵莹莹, 付欣豪, 陈庆悟, 杜晓华, 谭成侠. 吡啶联噻唑双酰胺类化合物的设计、合成及杀虫活性[J]. 有机化学, 2022, 42(11): 3870-3879. |
| [14] | 代阿丽, 张仁凤, 李传会, 余利娇, 王娅, 吴剑. 含三氟甲基吡啶酰胺结构的N-氰基磺酰亚胺衍生物的合成及生物活性[J]. 有机化学, 2021, 41(9): 3633-3642. |
| [15] | 何凤, 郭声鑫, 代阿丽, 张仁凤, 吴剑. 含三氟甲基吡啶结构的酰胺类衍生物的合成、表征及生物活性研究[J]. 有机化学, 2021, 41(8): 3303-3311. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||