有机化学 ›› 2025, Vol. 45 ›› Issue (5): 1460-1477.DOI: 10.6023/cjoc202405038 上一篇 下一篇
综述与进展
李顺曦a,b, 游力栩a,b, 李玉龙a,*( ), 舒伟a,b,*(
), 舒伟a,b,*( )
)
收稿日期:2024-05-27
修回日期:2024-07-02
发布日期:2024-08-16
基金资助:
Shunxi Lia,b, Lixu Youa,b, Yulong Lia,*( ), Wei Shua,b,*(
), Wei Shua,b,*( )
)
Received:2024-05-27
Revised:2024-07-02
Published:2024-08-16
Contact:
* E-mail: Supported by:文章分享

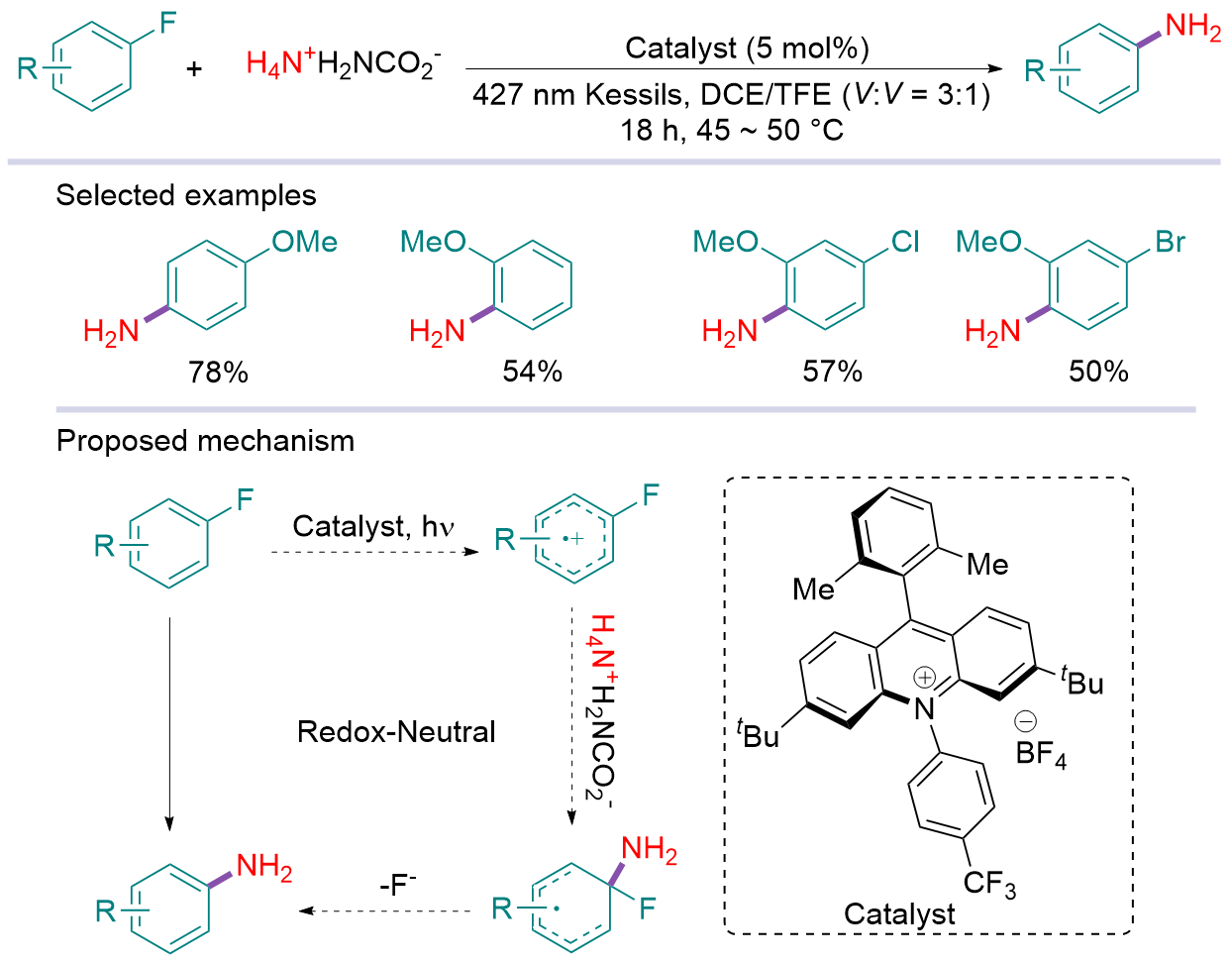
有机含氮化合物广泛存在于天然产物、药物分子和材料等功能分子中. 因此, 从简单易得的原料出发, 发展绿色、高效地构建C—N键的方法具有重要意义. 光是一种广泛存在且具有丰富易得、绿色经济和可再生等特点的自然资源. 另外, 氨及其等价体作为固氮的直接产物, 是一种极为经济、易得且稳定的含氮无机化合物, 也是一类理想的潜在氮来源. 将氨及其等价体中的氮原子高效转移到各种有机化合物中, 将间接实现固氮转化, 进而实现将氮气转移到有机化合物中. 因此, 利用光催化发展氨及其等价体参与的C—N成键反应具有重要的研究价值. 系统综述了近年来光介导氨及其等价体参与的碳氮成键反应进展, 并展望了氨、无机铵盐等在光催化下参与C—N成键的应用前景.
李顺曦, 游力栩, 李玉龙, 舒伟. 光介导氨及其等价体参与的碳氮成键反应研究进展[J]. 有机化学, 2025, 45(5): 1460-1477.
Shunxi Li, Lixu You, Yulong Li, Wei Shu. Recent Progress on Light-Mediated C—N Bond-Forming Processes from Ammonia and Equivalents[J]. Chinese Journal of Organic Chemistry, 2025, 45(5): 1460-1477.



| [1] |
|
|
(赵丽平, 刘连升, 胡岗, 骆美良, 黄美春, 严小倩, 中国临床药理学杂志, 2019, 35, 2255.)
|
|
| [2] |
(a)
pmid: 27809503 |
|
(b)
pmid: 27809503 |
|
|
(c)
pmid: 27809503 |
|
|
(d)
pmid: 27809503 |
|
| [3] |
(a)
pmid: 36121680 |
|
(b)
pmid: 36121680 |
|
|
(c)
pmid: 36121680 |
|
|
(d)
doi: 10.1021/acsnano.2c06059 pmid: 36121680 |
|
|
(e)
pmid: 36121680 |
|
| [4] |
(a)
doi: 10.1039/b820303b pmid: 23461586 |
|
(b)
doi: 10.1021/cr300272t pmid: 23461586 |
|
| [5] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
| [6] |
pmid: 27689804 |
| [7] |
(a)
pmid: 21391563 |
|
(b)
doi: 10.1021/cr1002276 pmid: 21391563 |
|
| [8] |
(a)
|
|
(b)
|
|
|
(c)
|
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
doi: 10.1021/ol401906q pmid: 23901827 |
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
doi: 10.1126/science.adk2312 pmid: 38175889 |
| [45] |
|
| [46] |
|
| [47] |
|
| [1] | 梁珍, 徐维焱, 陈毅, 邱化玉, 赵也哲, 沈佳斌, 王民. 铱催化N-芳基-2-氨基吡啶和碳酸亚乙烯酯合成吲哚衍生物[J]. 有机化学, 2025, 45(6): 2149-2156. |
| [2] | 高根伟, 李震, 李炎, 陆熹. 光/镍协同催化C(sp2)—C(sp3)键构建研究进展[J]. 有机化学, 2025, 45(6): 1905-1919. |
| [3] | 陈刚, 陈东, 聂广杰, 李林轩, 姚辉王能中, 黄年玉. 有机膦催化下橙酮衍生的氮杂二烯与氨基巴豆酸酯的[2+3]环加成反应[J]. 有机化学, 2025, 45(6): 2139-2148. |
| [4] | 谭永波, 舒洪波, 黄华文. 光诱导N-芳基丙烯酰胺参与的吲哚酮合成研究进展[J]. 有机化学, 2025, 45(6): 2086-2108. |
| [5] | 蒋晨阳, 尹艳丽, 江智勇. 光酶催化不对称自由基加成反应研究进展[J]. 有机化学, 2025, 45(5): 1614-1633. |
| [6] | 吴昊岩, 赫明月, 马军安, 张发光. 三芳胺在光催化反应中的研究进展[J]. 有机化学, 2025, 45(5): 1634-1643. |
| [7] | 吴利华, 杨建静, 闫克鲁, 许丽荣, 文江伟. 单原子光催化有机合成研究进展[J]. 有机化学, 2025, 45(5): 1591-1613. |
| [8] | 谭芳芳, 史孟欣, 张文敏, 李洋. 光催化生物质相关转化[J]. 有机化学, 2025, 45(5): 1523-1547. |
| [9] | 黄嘉浩, 黄雅豪, 胡鹏. 氢原子转移介导的光催化C(sp3)—H键氧化反应进展[J]. 有机化学, 2025, 45(5): 1509-1522. |
| [10] | 周思成, 刘运奎. P/N-杂配铜(I)光催化剂介导的可见光催化反应进展[J]. 有机化学, 2025, 45(5): 1644-1668. |
| [11] | 陈雨佳, 刘志林, 陈凯, 向皞月, 阳华. 无金属、光催化氧化苄基C—H键以获得羰基官能团[J]. 有机化学, 2025, 45(5): 1755-1762. |
| [12] | 林风, 张艳, 吴明, 刘会艳, 郝文娟, 姜波. 利用可见光引发1,6-烯炔的增环酰化双官能化制备1-茚酮衍生物[J]. 有机化学, 2025, 45(5): 1729-1738. |
| [13] | 洪洋, 邓红平. 可见光催化的酸性C(sp3)—H键官能团化反应研究进展[J]. 有机化学, 2025, 45(5): 1569-1590. |
| [14] | 牛丽菁, 吴成娟, 梁文静, 耿琰, 董育斌. 光催化串联反应构建共价有机框架[J]. 有机化学, 2025, 45(5): 1707-1715. |
| [15] | 谭燕, 应佳乐, 於兵, 陆展. 可见光促进烯基硅化合物有氧氧化-叠氮化反应[J]. 有机化学, 2025, 45(5): 1684-1690. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||