有机化学 ›› 2025, Vol. 45 ›› Issue (12): 4298-4314.DOI: 10.6023/cjoc202504026 上一篇 下一篇
综述与进展
邢德悦†, 王阳†, 房亭轩, 解瑞俊, 李潇, 贾慧劼*( ), 竺宁*(
), 竺宁*( )
)
收稿日期:2025-04-14
修回日期:2025-07-11
发布日期:2025-08-26
通讯作者:
贾慧劼, 竺宁
作者简介:† 共同第一作者
基金资助:
Deyue Xing, Yang Wang, Tingxuan Fang, Ruijun Xie, Xiao Li, Huijie Jia*( ), Ning Zhu*(
), Ning Zhu*( )
)
Received:2025-04-14
Revised:2025-07-11
Published:2025-08-26
Contact:
Huijie Jia, Ning Zhu
About author:† These authors contributed equally to this work
Supported by:文章分享

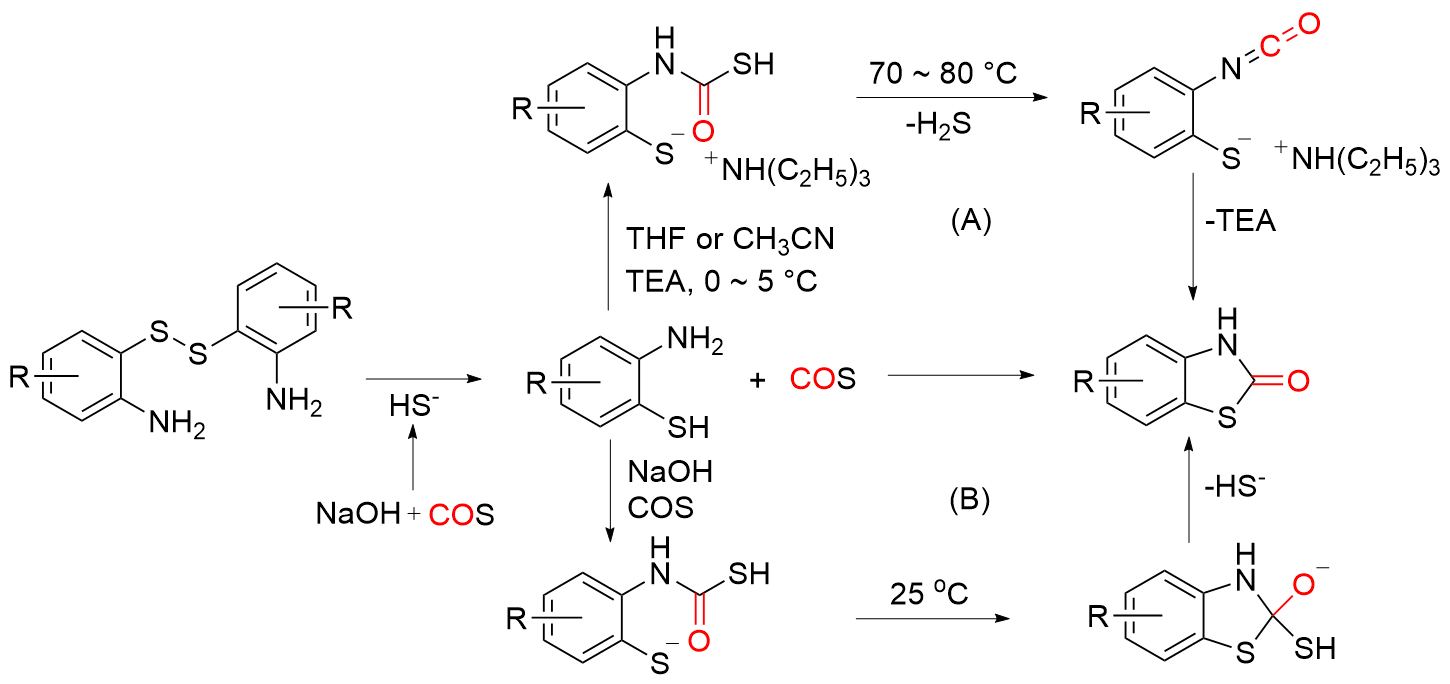
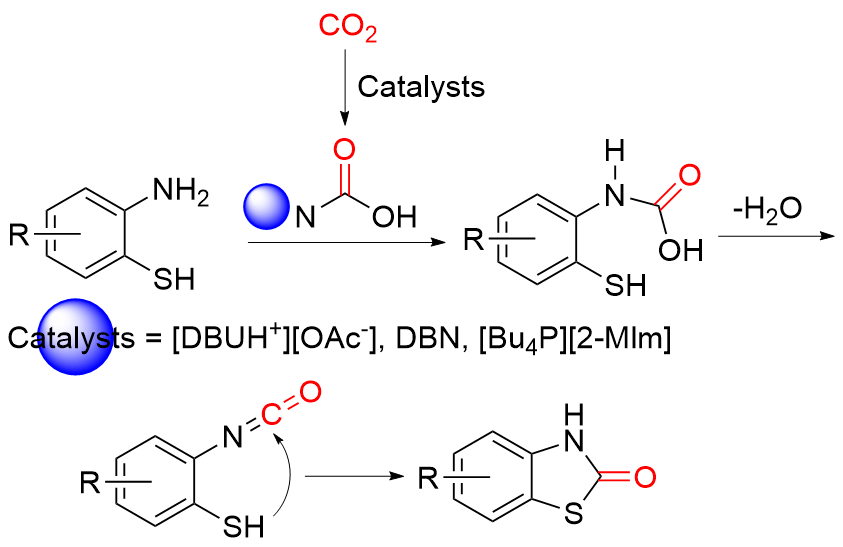
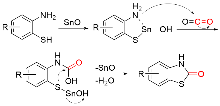
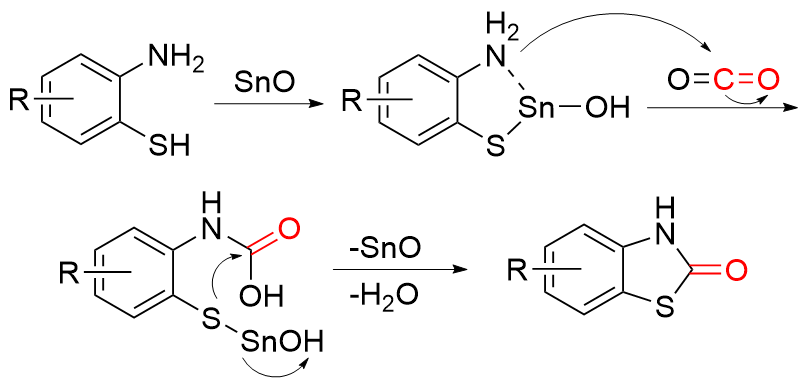
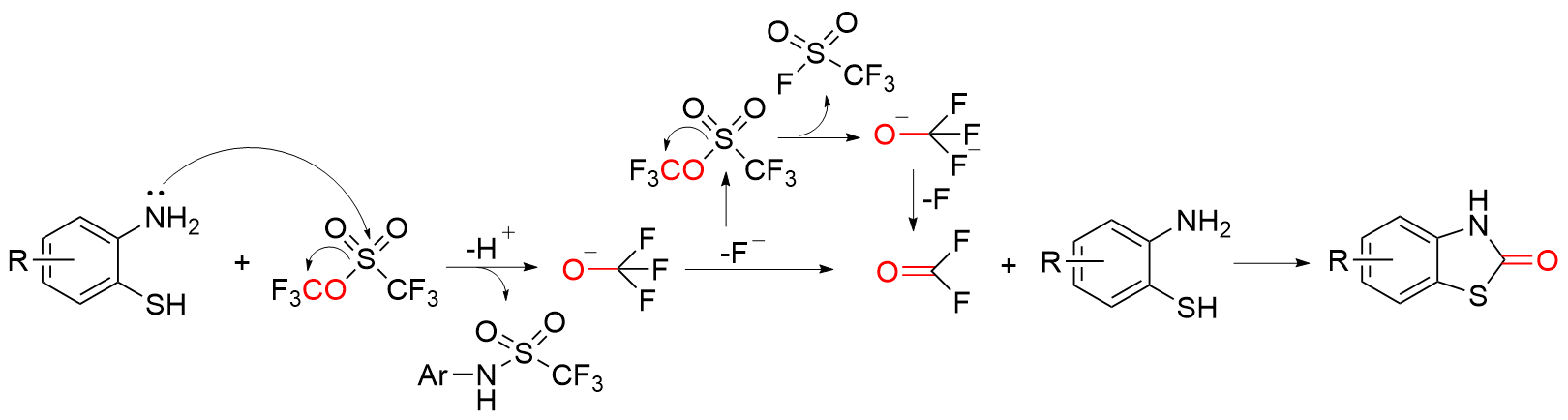
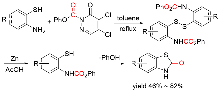
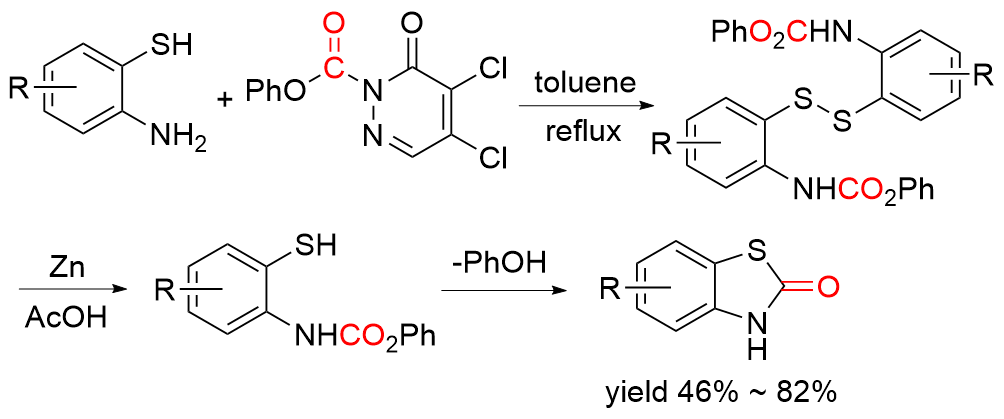
苯并噻唑-2-酮是一种含有苯环和硫氮杂环的稠环化合物, 具有广泛的生物活性, 在农业及医药领域有着较高的应用价值, 其合成方法的研究也备受关注. 本综述通过调研文献发现2-氨基苯硫酚、2-取代苯并噻唑以及邻卤苯胺是合成苯并噻唑-2-酮的主要原料, 并系统总结了这三种原料合成苯并噻唑-2-酮的方法、路线及其反应机理, 为该类化合物的合成提供了参考依据. 此外, 本综述还探讨了羰基化试剂的结构特点、反应活性以及原子经济性, 为羰基化试剂在有机合成中的应用提供了理论依据.
邢德悦, 王阳, 房亭轩, 解瑞俊, 李潇, 贾慧劼, 竺宁. 苯并噻唑-2-酮类化合物的合成研究进展[J]. 有机化学, 2025, 45(12): 4298-4314.
Deyue Xing, Yang Wang, Tingxuan Fang, Ruijun Xie, Xiao Li, Huijie Jia, Ning Zhu. Progress in the Synthesis of Benzothiazole-2-ones[J]. Chinese Journal of Organic Chemistry, 2025, 45(12): 4298-4314.
| Structure | Molecular weight | State of matter at room temperature | Carbonylation mechanism | Mass ratio of carbonyl groups in the reagent |
|---|---|---|---|---|
| | 27.99 | Gas | CO undergoes in-situ conversion to COS/Se for participation in subsequent reactions or engages in direct reactions mediated by metal catalysis | 100% |
| | 43.99 | Gas | The carbonyl group is attacked by a nucleophile to form an amide or ester, which then participates in subsequent reactions | 63.6% |
| | 59.97 | Gas | The thiocarbonyl group is attacked by an amine nucleophile, forming thiocarbamate intermediates that subsequently engage in further reactions | 46.7% |
| | 97.93 | Gas | The acyl chloride is attacked by an amine nucleophile, forming carbamyl chloride that subsequently engage in further reactions | 28.6% |
| | 293.80 | Solid | Triphosgene cleaves into 3 equiv. of phosgene, which then participate in the reaction | 28.6% |
| | 65.99 | Gas | The acyl fluoride is attacked by an amine nucleophile, forming carbamyl fluoride that subsequently engage in further reactions | 42.4% |
| | 217.95 | Gas | The O—S bond cleavage yielded OCF3 and F, generating acyl fluoride, which then participated in the reaction | 12.8% |
| | 108.00 | Liquid | The acyl chloride is attacked by an amine nucleophile, forming carbamate that subsequently engage in further reactions | 25.9% |
| | 60.03 | Solid | The urea is attacked by an amine nucleophile, forming another urea that subsequently engage in further reactions | 46.6% |
| | 256.03 | Solid | The electron-withdrawing inductive effect reduces the conjugation of the ester bond, facilitating the cleavage of the C—O bond to yield the carbonyl group | 11.0% |
| | 382.04 | Solid | The conjugation effect of the aromatic ring reduces the conjugation of the C—S bond in the thioester, promoting the cleavage of the C—S bond to form the carbonyl group | 7.4% |
| | 162.05 | Solid | The conjugation effect of the aromatic ring reduces the bond energy of the C—N bond in the amide, facilitating the cleavage of the C—N bond to yield the carbonyl group | 17.2% |
| | 283.98 | Liquid | The conjugation effect of the double bond facilitates the cleavage of the C—N bond in the amide, while the conjugation effect of the aromatic ring reduces the conjugation of the ester bond, promoting the cleavage of both the C—N and C—O bonds to yield the carbonyl group | 9.8% |
| | 119.04 | Liquid | The carbon atom in isocyanate, influenced by the electron-withdrawing inductive effects of both the oxygen and nitrogen atoms, is highly susceptible to nucleophilic attack, leading to the formation of urea or carbamate | 23.5% |
| Structure | Molecular weight | State of matter at room temperature | Carbonylation mechanism | Mass ratio of carbonyl groups in the reagent |
|---|---|---|---|---|
| | 27.99 | Gas | CO undergoes in-situ conversion to COS/Se for participation in subsequent reactions or engages in direct reactions mediated by metal catalysis | 100% |
| | 43.99 | Gas | The carbonyl group is attacked by a nucleophile to form an amide or ester, which then participates in subsequent reactions | 63.6% |
| | 59.97 | Gas | The thiocarbonyl group is attacked by an amine nucleophile, forming thiocarbamate intermediates that subsequently engage in further reactions | 46.7% |
| | 97.93 | Gas | The acyl chloride is attacked by an amine nucleophile, forming carbamyl chloride that subsequently engage in further reactions | 28.6% |
| | 293.80 | Solid | Triphosgene cleaves into 3 equiv. of phosgene, which then participate in the reaction | 28.6% |
| | 65.99 | Gas | The acyl fluoride is attacked by an amine nucleophile, forming carbamyl fluoride that subsequently engage in further reactions | 42.4% |
| | 217.95 | Gas | The O—S bond cleavage yielded OCF3 and F, generating acyl fluoride, which then participated in the reaction | 12.8% |
| | 108.00 | Liquid | The acyl chloride is attacked by an amine nucleophile, forming carbamate that subsequently engage in further reactions | 25.9% |
| | 60.03 | Solid | The urea is attacked by an amine nucleophile, forming another urea that subsequently engage in further reactions | 46.6% |
| | 256.03 | Solid | The electron-withdrawing inductive effect reduces the conjugation of the ester bond, facilitating the cleavage of the C—O bond to yield the carbonyl group | 11.0% |
| | 382.04 | Solid | The conjugation effect of the aromatic ring reduces the conjugation of the C—S bond in the thioester, promoting the cleavage of the C—S bond to form the carbonyl group | 7.4% |
| | 162.05 | Solid | The conjugation effect of the aromatic ring reduces the bond energy of the C—N bond in the amide, facilitating the cleavage of the C—N bond to yield the carbonyl group | 17.2% |
| | 283.98 | Liquid | The conjugation effect of the double bond facilitates the cleavage of the C—N bond in the amide, while the conjugation effect of the aromatic ring reduces the conjugation of the ester bond, promoting the cleavage of both the C—N and C—O bonds to yield the carbonyl group | 9.8% |
| | 119.04 | Liquid | The carbon atom in isocyanate, influenced by the electron-withdrawing inductive effects of both the oxygen and nitrogen atoms, is highly susceptible to nucleophilic attack, leading to the formation of urea or carbamate | 23.5% |
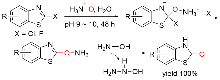
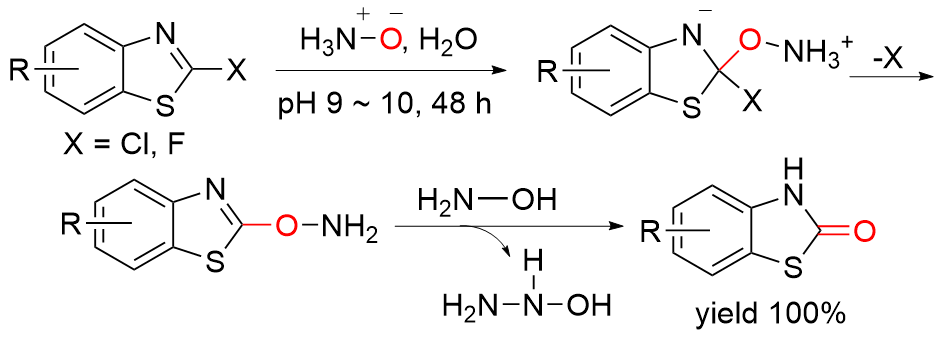
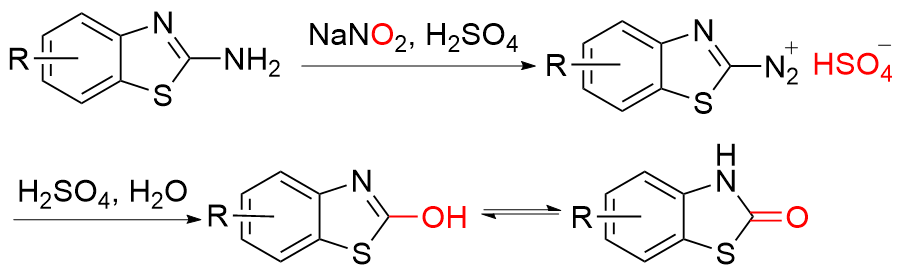

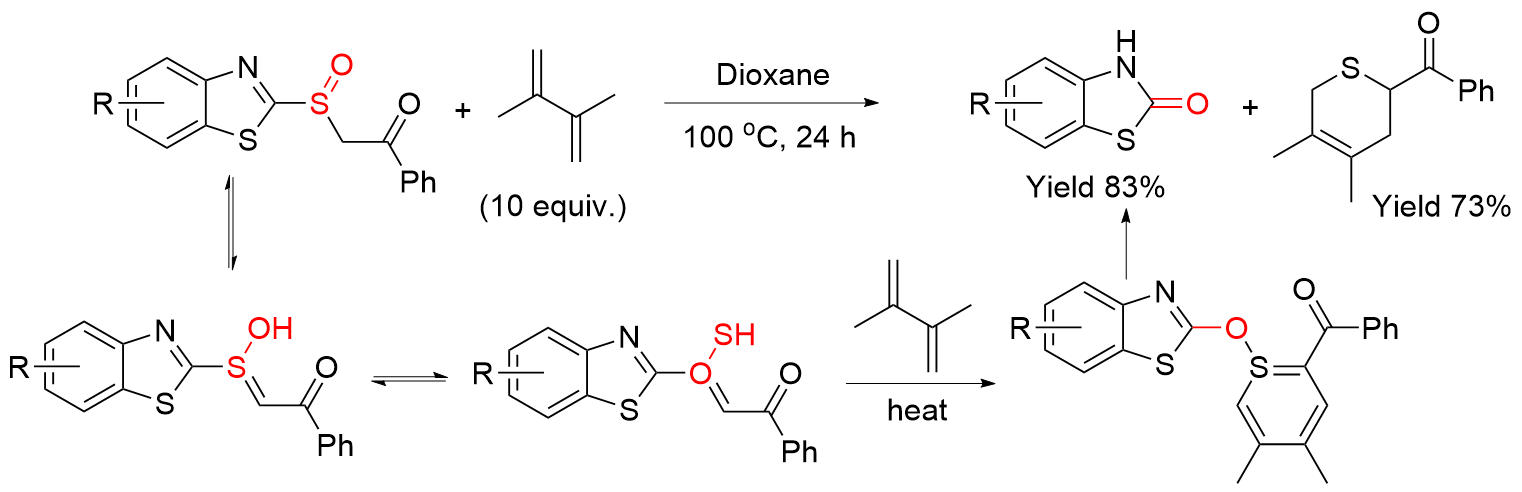
| [5] |
pmid: 3759941 |
| [6] |
doi: 10.3906/kim-1912-55 pmid: 33488213 |
| [7] |
pmid: 9544213 |
| [8] |
pmid: 16209513 |
| [9] |
doi: 10.1246/bcsj.77.169 |
| [10] |
doi: 10.1039/d0nj00273a |
| [11] |
doi: 10.1002/cber.v43:2 |
| [12] |
doi: 10.1002/ejoc.v2020.4 |
| [13] |
doi: 10.1016/S0040-4039(00)72336-7 |
| [14] |
doi: 10.1002/hc.v5:5/6 |
| [15] |
|
| [16] |
|
|
(时广辉, 杜云哲, 高媛媛, 贾慧劼, 洪海龙, 韩利民, 竺宁, 有机化学, 2023, 43, 491.)
doi: 10.6023/cjoc202207029 |
|
| [17] |
doi: 10.1016/j.tetlet.2011.06.049 |
| [18] |
doi: 10.1021/acs.joc.3c02140 |
| [19] |
|
| [20] |
doi: 10.1002/jhet.v23:3 |
| [21] |
doi: 10.1021/cs400256j |
| [22] |
doi: 10.1002/asia.v11.19 |
| [23] |
doi: 10.3390/catal8070271 |
| [24] |
|
| [25] |
doi: 10.1134/S1990793119080062 |
| [26] |
|
| [27] |
doi: 10.1002/anie.201915414 pmid: 31995256 |
| [28] |
doi: 10.1002/chem.v25.46 |
| [29] |
doi: 10.1002/anie.201208741 pmid: 23653429 |
| [30] |
|
| [31] |
doi: 10.1134/S107042801109020X |
| [32] |
doi: 10.1021/ja00853a038 |
| [33] |
doi: 10.1055/s-00000175 |
| [34] |
doi: 10.1007/s11030-015-9572-8 |
| [35] |
doi: 10.1208/s12248-011-9311-8 pmid: 22183188 |
| [36] |
doi: 10.1055/s-00000083 |
| [37] |
doi: 10.1080/00397918208063680 |
| [38] |
doi: 10.1248/cpb.37.2334 |
| [39] |
doi: 10.1039/C6GC03053J |
| [40] |
doi: 10.1007/s11426-014-5149-0 |
| [41] |
doi: 10.1016/j.bmc.2007.07.008 |
| [42] |
|
| [43] |
doi: 10.1002/jhet.v42:4 |
| [44] |
doi: 10.1021/op049953y |
| [45] |
doi: 10.1021/acs.orglett.1c00893 |
| [46] |
doi: 10.1021/jo00180a009 |
| [47] |
pmid: 2955118 |
| [48] |
doi: 10.1039/c2ob26929e pmid: 23224221 |
| [49] |
doi: 10.1039/D1GC04361G |
| [50] |
doi: 10.1002/jhet.v25:4 |
| [51] |
doi: 10.1002/anie.v51.19 |
| [52] |
doi: 10.1002/ejoc.v27.1 |
| [53] |
doi: 10.1021/jacs.6b03855 |
| [54] |
doi: 10.1016/j.tet.2007.01.043 |
| [55] |
doi: 10.1246/bcsj.65.3163 |
| [56] |
|
| [57] |
doi: 10.1016/S0040-4020(02)01365-0 |
| [58] |
doi: 10.1016/j.tetlet.2012.03.006 |
| [59] |
doi: 10.1039/c3ra46803h |
| [60] |
doi: 10.1016/j.ejmech.2008.09.010 |
| [61] |
doi: 10.3987/COM-92-6193 |
| [62] |
doi: 10.1021/ol7015737 |
| [63] |
|
| [64] |
doi: 10.1016/j.inoche.2021.108590 |
| [65] |
doi: 10.1039/D0GC03723K |
| [66] |
doi: 10.1021/acs.joc.2c01060 |
| [1] |
doi: 10.3390/molecules17010989 |
| [2] |
doi: 10.1021/es052205j |
| [3] |
doi: 10.1021/acsmedchemlett.9b00330 pmid: 32184960 |
| [4] |
|
| [67] |
doi: 10.1002/adsc.v364.1 |
| [68] |
doi: 10.1081/SCC-120027722 |
| [1] | 田灵燕, 王守锋, 曾伟. 大蒜中S-1-丙烯基-L-半胱氨酸的合成及生物学特性研究进展[J]. 有机化学, 2026, 46(1): 74-86. |
| [2] | 刘小宇, 许庭瑞, 秦勇. 吗啡类生物碱的全合成研究新进展★[J]. 有机化学, 2025, 45(9): 3098-3112. |
| [3] | 林天星, 刘天飞. N-三氟甲基酰胺合成方法的研究进展[J]. 有机化学, 2025, 45(6): 1995-2006. |
| [4] | 李闯, 张成, 刘小宇, 秦勇. 二萜生物碱全合成研究进展[J]. 有机化学, 2025, 45(3): 881-895. |
| [5] | 郭国菊, 吴启龙, 董宇振, 张洁, 石永佳, 柳清, 杨道山. 光/电化学驱动硫代磷酸酯类化合物的合成研究进展[J]. 有机化学, 2025, 45(10): 3719-3740. |
| [6] | 何卫保, 易荣楠, 杨梓, 伍智林, 何卫民. Langlois试剂在电化学三氟甲基化反应中的应用进展[J]. 有机化学, 2025, 45(10): 3534-3545. |
| [7] | 刘艺琦, 陈文婕, 赵泽艳, 王苏棋, 唐圣松, 何卫民, 彭俊梅. 关于8种含氮杂环光催化合成的研究进展[J]. 有机化学, 2025, 45(10): 3546-3586. |
| [8] | 周岑, 赵新, 张霄. 三聚苯环化反应的研究进展[J]. 有机化学, 2025, 45(1): 42-55. |
| [9] | 曹素芳, 刘云云, 万结平. α-三氟甲基酮的合成及其脱氟转化反应研究进展[J]. 有机化学, 2025, 45(1): 86-103. |
| [10] | 张雅芳, 黄轩, 林琪, 钟海琼, 翁志强, 吴伟. 噻唑类化合物的合成研究进展[J]. 有机化学, 2024, 44(5): 1458-1479. |
| [11] | 关丽, 周艳艳, 毛永爆, 付恺森, 关文惠, 付义乐. 甲川链修饰菁染料的合成研究进展[J]. 有机化学, 2023, 43(8): 2682-2698. |
| [12] | 刘悦灵, 钟欣欣, 张干兵. Pd(0)催化1-R-3-苯基亚丙基环丙烷(R=Me/H)与呋喃甲醛[3+2]环加成反应机理的密度泛函理论研究[J]. 有机化学, 2023, 43(2): 660-667. |
| [13] | 刘婷婷, 胡宇才, 沈安. 亚胺配体协同氮杂环卡宾钯配合物催化碳碳偶联反应的作用机制[J]. 有机化学, 2023, 43(2): 622-628. |
| [14] | 宋树勇, 徐森苗. 三氟甲基烯烃的选择性C-F键活化最新进展[J]. 有机化学, 2023, 43(2): 411-425. |
| [15] | 刘鹏, 钟富明, 廖礼豪, 谭伟强, 赵晓丹. 炔烃参与的去芳构化反应构建螺环己二烯酮类化合物的研究进展[J]. 有机化学, 2023, 43(12): 4019-4035. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||