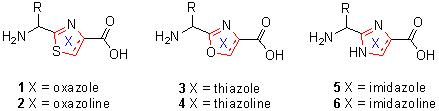
| 1 Sohda, K.; Nagai, K.; Yamori, T.; Suzuki, K.; Tanaka, A. J. Antibiot., 2005, 58, 27-31.2 Kanoh, K.; Matsuo, Y.; Adachi, K.; Imagawa, H.; Nishizawa, M.; Shizuri, Y. J. Antibiot. 2005, 58, 289-292.3 Wagner, B.; Schumann, D.; Linne, U.; Koert, U.; Marahiel, M. A. J. Am. Chem. Soc. 2006, 128, 10513-10520.4 Abbenante, G.; Fairlie, D. P.; Gahan, L.R.; Hanson, G. R.; Pierens, G. K.; van den Brenk, A. L. J. Am. Chem. Soc. 1996, 118, 10384-10388.5 Ishida, T.; Tanaka, M.; Nabae, M. I.; Inoue, M.; Kato, S. J.; Hamada, Y.; Shioiri, T. J. Org. Chem. 1988, 53, 107-112.6 Kaul, R.; Surprenant, S.; Lubell, W. D. J. Org. Chem. 2005, 70, 3838-3844.7 Hanessian, S.; McNaughton-Smith, G.; Lombart, H. G.; Lubell, W. D. Tetrahedron 1997, 53, 12789-12854. 8 Roy, R. S.; Gehring, A. M.; Milne, J. C.; Peter J. Belshaw, P. J.; Walsh, C. T. Nat. Prod. Rep. 1999, 16, 249-263.9 Somogyi, L.; Haberhauer, G.; Rebek, J. J. Tetrahedron 2001, 57, 1699-1708.10 Michael, J. P.; Pattenden, G. Angew. Chem. Int. Ed. Engl. 1993, 1, 105-106.11 Foster, M. P.; Concepcion, G. P.; Caraan, G. B.; Ireland, C. M. J. Org. Chem. 1992, 57, 6671-6675.12 Rudi, A.; Chill, L.; Aknin, M.; Kashman, Y. J. Nat. Prod. 2003, 66, 575-577.13 Perez, L. J.; Faulkner, D.J. J. Nat. Prod. 2003, 66, 247-250.14 Williams, A. B.; Jacobs, R. S. Cancer Lett. 1993, 71, 97-102 .15 Milligan, K. E.; Marquez, B. L.; Williamson, R. T.; Gerwick, W. H. J. Nat. Prod. 2000, 63, 1440-1443.16 Walke, M. A.; Heathcock, C. H. J. Org. Chem. 1992, 57, 5566-5568. 17 Cross, D. F. W.; Kenner, G. W.; Sheppard, R. C.; Stehr, C. E. J. chem. soc. 1963, 2143-2150.18 George R. Pettit, G. R.; Paul S. Nelson, P. S.; Holzapfel, C. W. J. Org. Chem. 1985, 50, 2654-2659.19 Holzapfel, C. W.; Pettit, G. R. J. Org. Chem. 1985, 50, 2323-2327.20 Schmidt, U.; Utz, R. Angew. Chem. Int. Ed. Engl. 1984, 23, 725-726.21 Kelly, R. C.; Gebhard, I.; Wicnienski, N. J. Org. Chem. 1986, 51, 4590-4594.22 Stankova, I. G.; Videnov, G. I.; Golovinsky, E. V.; Jung, G. J. Pept Sci. 1999, 5, 392-398.23 Houssin, R.; MichMe Lohez, M.; Bernier, J.-L.; Hgnichart, J.-P. J. Org. Chem. 1985, 50, 2787-2788.24 Yonezawa, Y.; Tani, N.; Shin, C.-g. Bull. Chem. Soc. Jpn. 2005, 78, 1492-1499.25 Burgess, E. M.; Penton, H. R.; Taylor, E. A. J. Org. Chem. 1973, 38, 26-31.26 Nemoto, H.; Kubota, Y.; Yamamoto, Y. J. Org. Chem. 1990, 55, 4515-4516.27 Claremon, D. A.; Phillips, B. T. Tetrahedron Lett. 1988, 29, 2155-2158.28 Burgess, E. M.; Penton, H. R.; Taylor, E. A.; Williams, W. M. Org. Synth. 1977, 56, 40. 1988, Coll. Vol. 6, 788.29 Wipf, P.; Fritch, P. C. Tetrahedron Lett. 1994, 35, 5397-5400.30 Hamada, Y.; Shibata, M.; Sugiuri, T.; Kato, S.; Shioiri, T. J. Org. Chem. 1987, 52, 1252-1255. 31 Martin, L. M.; Hu, B.-H. Tetrahedron Lett. 1999, 40, 7951-7953.32 Veale, E. B.; O’Brien, J. E.; Thomas McCabe, T.; Gunnlaugsson, T. Tetrahedron 2008, 64, 6794-6800.33 Credico, B. D.; Reginato, G.; Gonsalvi, L.; Peruzzini, M.; Rossin, A. Tetrahedron 2011, 67 , 267-27434 North, M.; Pattenden, G. Tetrahedron 1990, 46, 8267-8290.35 Boden, C. D. J.; Pattenden, G.; Ye, T. Synlett. 1995, 417-419.36 Raman, P.; Razavi, H.; Kelly, J. W. Org. Lett. 2000, 2, 3289-3292. 37 You, S.-L.; Razavi, H.; Kelly, J.-W. Angew. Chem. Int. Ed. 2003, 42, 83-85.38 Videnov, G.; Kaiser, D.; Kempter, C.; Jung, G. Angew. Chem. Int. Ed. Engl. 1996, 35, 1503-1506.39 Stanchev, M.; Tabakova, S.; Videnov, G.; Golovinsky, E.; Jung, G. Arch. Pharm. Pharm. Med. Chem. 1999, 332, 297-304.40 Wipf, P.; Miller, C. P. Tetrahedron Lett. 1992, 33, 907-910.41 Bose, A. K.; Manhas. M. S.; Sahu, D. P.; Hegde, V. R. Can. J. Chem. 1984, 62, 2498-2505.42 Krook, M. A.; Miller, M. J. J. Org. Chem. 1985, 50, 1126-112843 Phillips, A. J.; Uto, Y.; Wipf, P.; Reno, M. J.; Williams, D. R. Org. Lett. 2000, 2, 1165-1168.44 Dew, D. B.; Martii, J. C. J. Am. Chem. Soc. 1991, 113, 7277-7287.45 Ireland, R. E.; Liu, L. J. Org. Chem. 1993, 58, 2899.46 Wipf, P.; Miller, C. P. J. Org. Chem. 1993, 58, 3604-3606.47 Somogyi, L.; Haberhauer, G.; Rebek, J. J. Tetrahedron 2001, 57, 1699-1708.48 Shibata, K.; Yoshida, M.; Doi, T.; Takahashi, T. Tetrahedron Lett. 2010, 51, 1674-1677.49 Jeong, S.; Chen, X.; Harran, P. G. J. Org. Chem. 1998, 63, 8640-8641.50 Haberhauer, G.; Rominger, F. Tetrahedron Lett. 2002, 43, 6335-6338.51 You, S.-L.; Kelly, W. K. Org. Lett. 2004, 6, 1681-1683.52 Haberhauer, G.; Rominger, F. Tetrahedron Lett. 2002, 43, 6335-6338.53 Haberhauer, G.; Rominger, F. Eur. J. Org. Chem. 2003, 3209-3218. |