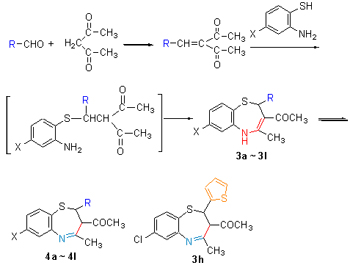
| [1] Fabrizio, M.; Fabio, D.; Aldo, F.; Merlo, G. J. Comb. Chem. 2001, 3, 224.[2] Prakash, O.; Kumar, A.; Sadana, A.; Prakash, R.; Singh, S. P.; Claramunt, R. M.; Sanz, D.; Alkorta, I.; Elguero, J. Tetrahedron 2005, 61, 6642.[3] Nagao, T.; Sato, M.; Iwasawa, Y.; Takada, T.; Ishida, R. Jpn. J. Pharmacol. 1972, 22, 467.[4] Nagao, T.; Sato, M.; Nakajima, H.; Kiyomoto, A. Chem. Pharm. Bull. 1973, 21, 92.[5] Yamada, K.; Shimamura, T.; Nakajima, H. Jpn. J. Pharmacol. 1973, 23, 321.[6] Bariwal, J. B.; Upadhyay, K. D.; Manvar, A. T.; Trivedi, J. C.; Singh, J. S.; Jain, K. S.; Shah, A. K. Eur. J. Med. Chem. 2008, 43, 2279.[7] Grandolini, G.; Perioli, L.; Ambrogi, V. Eur. J. Med. Chem. 1999, 34, 701.[8] Bajaj, K.; Archana; Kumar, A. Eur. J. Med. Chem. 2004, 39, 369.[9] Ambrogi, V.; Furlani, A.; Grandolini, G.; Papaioannou, A.; Perioli, L.; Scarcia, V.; Tuttobello, L. Eur. J. Med. Chem. 1993, 28, 659.[10] Maruenda, H.; Johnson, F. J. Med. Chem. 1995, 38, 2145.[11] Roberto. S.; Roberta. C. Farmaco 2005, 60, 385.[12] Zhang, P.; Wang, L.-Z.; Wu, H.-S.; Lan, J.-M.; Li, Y.; Wang, Y.-X. Chin. Chem. Lett. 2009, 20, 660 (in Chinese). (张萍, 王兰芝, 吴惠姝, 蓝佳明, 李媛, 王永祥, 中国化学快报, 2009, 20, 660.)[13] Li, W.-H.; Lan, J.-M.; Li, C.; Cao, Y.-T.; Wang, H.-B.; Wang, L.-Z.; Zhang, P.; Wang, Y.-X.; Li, Y. Acta Chim. Sinica 2009, 67, 2732 (in Chinese). (李文红, 兰佳明, 李成, 曹云涛, 王海波, 王兰芝, 张萍, 王永祥, 李媛, 化学学报, 2009, 67, 2732.)[14] Zhang, P.; Xuan, Y.; Liu, W.; Li, Y. Chin. J. Org. Chem. 2011, 31, 1240 (in Chinese). (张萍, 宣烨, 刘薇, 李媛, 有机化学, 2011, 31, 1240.)[15] Zhang, P.; Huang, J. H.; Wang, L.-Z.; Liu, W.; Li, Y. Chin. J. Org. Chem. 2010, 30, 1939 (in Chinese). (张萍, 黄金华, 王兰芝, 刘薇, 李媛, 有机化学, 2010, 30, 1939.)[16] Li, W.-H.; Zhang, L. F.; Liu, W.; Du, X. Q.; Li, Y. Chin. J. Org. Chem. 2011, 31, 1252 (in Chinese). (李文红, 张玲芬, 刘薇, 杜星琼, 李媛, 有机化学, 2011, 31, 1252.)[17] Wang, L.-Z.; Zhang, P.; Zhang, X.-M.; Zhang, Y.-H.; Wang, Y.-X.; Li, Y. Eur. J. Med. Chem. 2009, 44, 2815.[18] Qiu, S. L.; Wang, L.-Z.; Li, W.-H.; Li, Y. Acta Chim. Sinica 2011, 69, 1217 (in Chinese). (邱召来, 王兰芝, 李文红, 李媛, 化学学报, 2011, 69, 1217.)[19] Hooton, T. M. Infect. Dis. Clin. North. Am. 2003, 17, 303.[20] Gallo-Martínez, L.; Campín-Falcó, P.; Sevillano-Cabeza, A. J. Pharm. Biomed. Anal. 2002, 29, 405.[21] Liu, M.; Zhang, W.-J.; Gao, N. Chem. Reag. 2009, 31, 795 (in Chinese). (刘敏, 章文军, 高宁, 化学试剂, 2009, 31, 795.)[22] Xing, Y.; Xie, Z.-F.; Liu, F.-M. Chem. J. Chin. Univ. 2008, 29, 533 (in Chinese). (邢烨, 解正峰, 刘方明, 高等学校化学学报, 2008, 29, 533.)[23] Yang, D. B.; Liu, F. M.; Shen, S. W.; Chen, S. Q. Chin. J. Org. Chem. 2010, 30, 244 (in Chinese). (杨德保, 刘方明, 沈松伟, 陈盛钦, 有机化学, 2010, 30, 244.)[24] Nishiyama, Y.; Makino, Y.; Hamanaka, S.; Ogawa, A.; Sonoda, N. Bull. Chem. Soc. Jpn. 1989, 62, 1683. |