有机化学 ›› 2021, Vol. 41 ›› Issue (5): 1925-1938.DOI: 10.6023/cjoc202009035 上一篇 下一篇
综述与进展
收稿日期:2020-09-15
修回日期:2020-11-12
发布日期:2020-12-01
通讯作者:
葛丹华, 褚雪强
基金资助:
Buqing Chenga, Danhua Gea,*( ), Xin Wangb, Xueqiang Chua,*(
), Xin Wangb, Xueqiang Chua,*( )
)
Received:2020-09-15
Revised:2020-11-12
Published:2020-12-01
Contact:
Danhua Ge, Xueqiang Chu
About author:Supported by:文章分享

有机氟化学和杂环化学的研究对药物研发、新材料制备等方面具有重要意义. 过去几年中, 利用高度商业化、低毒性、廉价、稳定、易操作的全氟烷基卤化物为含氟砌块构建氟烷基取代杂环化合物取得了重要进展. 本综述将全氟烷基卤化物作为含氟砌块参与构建杂环化合物的报道进行总结: (1)通过全氟烷基卤化物的单个碳卤键官能化构建杂环化合物; (2)通过全氟烷基卤化物的多个碳卤键官能化构建杂环化合物. 通过全氟烷基卤化物产生全氟烷基自由基(?Rf)继而对不饱和类型底物进行加成-环化串联, 将含氟基团引入杂环, 合成了一系列氟烷基取代杂环化合物, 如喹氧啉、菲蒽、肼、噻唑、嘧啶、偶氮三环、异噁唑等. 本文旨在为从事有机杂环合成和有机氟化学的研究人员提供参考和启示, 从而有助于新的含氟砌块和新的氟化反应的设计开发.
程步清, 葛丹华, 汪欣, 褚雪强. 全氟烷基卤化物作为含氟砌块在构建氟烷基取代杂环化合物中的研究进展[J]. 有机化学, 2021, 41(5): 1925-1938.
Buqing Cheng, Danhua Ge, Xin Wang, Xueqiang Chu. Perfluoroalkyl Halides as Fluorine-Containing Building Blocks for the Synthesis of Fluoroalkylated Heterocycles[J]. Chinese Journal of Organic Chemistry, 2021, 41(5): 1925-1938.
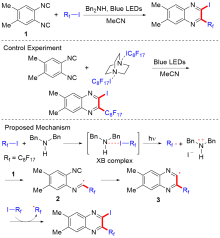

| [1] |
(a) Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432.
doi: 10.1021/cr4002879 pmid: 32891056 |
|
(b) Prchalová, E.; Štěpánek, O.; Smrček, S.; Kotora, M. Future Med. Chem., 2014, 6, 1201.
doi: 10.4155/fmc.14.53 pmid: 32891056 |
|
|
(c) Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. iScience 2020, 23, 101467.
doi: S2589-0042(20)30659-3 pmid: 32891056 |
|
| [2] |
For selected reviews, see: (a) Ni C.; Hu M.; Hu J. Chem. Rev. 2015, 115, 765.
doi: 10.1021/cr5002386 |
|
(b) Ni, C.; Hu, J. Chem. Soc. Rev. 2016, 45, 5441.
doi: 10.1039/C6CS00351F |
|
| [3] |
For selected examples, see: (a) Shao X.-X.; Xu C.-F.; Lu L.; Shen Q. Acc. Chem. Res. 2015, 48, 1227.
doi: 10.1021/acs.accounts.5b00047 |
|
(b) Li, S.; Ma, J.-A. Chem. Soc. Rev. 2015, 44, 7439.
doi: 10.1039/C5CS00342C |
|
|
(c) Feng, Z.; Xiao, Y.-L.; Zhang, X. Acc. Chem. Res. 2018, 51, 2264.
doi: 10.1021/acs.accounts.8b00230 |
|
|
(d) Huang, W.; Hu, M.; Wan, X.; Shen, Q. Nat. Commun. 2019, 10, 2963.
doi: 10.1038/s41467-019-10851-4 |
|
|
(e) Fu, X.-P.; Xue, X.-S.; Zhang, X.-Y.; Xiao, Y.-L.; Zhang, S.; Guo, Y.-L.; Leng, X.; Houk, K. N.; Zhang, X. Nat. Chem. 2019, 11, 948.
doi: 10.1038/s41557-019-0331-9 |
|
| [4] |
For selected reviews, see: (a) Hu J; Ding K Acta Chim. Sinica 2018, 76, 905. (in Chinese).
doi: 10.6023/A1812E001 |
|
(胡金波, 丁奎岭, 化学学报, 2018, 76, 905.)
doi: 10.6023/A1812E001 |
|
|
(b) Ren, Z.; Ren, N.; Zhang, F.; Ma, J. Acta Chim. Sinica 2018, 76, 940. (in Chinese).
doi: 10.6023/A18070279 |
|
|
(任智雯, 任楠, 张发光, 马军安, 化学学报, 2018, 76, 940.)
doi: 10.6023/A18070279 |
|
|
(c) Liu, Q.; Zhao, X.; Li, J.; Cao, S. Acta Chim. Sinica 2018, 76, 945. (in Chinese).
doi: 10.6023/A18080322 |
|
|
(刘青云, 赵祥虎, 李佳录, 曹松, 化学学报, 2018, 76, 945.)
doi: 10.6023/A18080322 |
|
| [5] |
For selected reviews, see: (a) Ni C.; Hu J. Chem. Soc. Rev. 2016, 45, 5441.
doi: 10.1039/C6CS00351F |
|
(b) Pan, X.; Xia, H.; Wu, J. Org. Chem. Front. 2016, 3, 1163.
doi: 10.1039/C6QO00153J |
|
|
(c) Song, H.-X.; Han, Q.-Y.; Zhao, C.-L.; Zhang, C.-P. Green Chem. 2018, 20, 1662.
doi: 10.1039/C8GC00078F |
|
|
(d) Chu, X.-Q.; Ge, D.; Shen, Z.-L.; Loh, T.-P. ACS Catal. 2018, 8, 258.
doi: 10.1021/acscatal.7b03334 |
|
| [6] |
For selected reviews, see: (a) Koike T.; Akita M. Chem 2018, 4, 409.
doi: 10.1016/j.chempr.2017.11.004 |
|
(b) Barata-Vallejo, S.; Cooke, M. V.; Postigo, A. ACS Catal. 2018, 8, 7287.
doi: 10.1021/acscatal.8b02066 |
|
| [7] |
Sun, X.; Wang, W.; Li, Y.; Ma, J.; Yu, S. Org. Lett. 2016, 18, 4638.
doi: 10.1021/acs.orglett.6b02271 |
| [8] |
Wang, Y.; Wang, J.; Li, G.-X.; He, G.; Chen, G. Org. Lett. 2017, 19, 1442.
doi: 10.1021/acs.orglett.7b00375 |
| [9] |
Yu, J.-M.; Cai, C. Eur. J. Org. Chem. 2017,6008.
|
| [10] |
Zheng, J.; Chen, P.; Yuan, Y.; Cheng, J. J. Org. Chem. 2017, 82, 5790.
doi: 10.1021/acs.joc.7b00598 |
| [11] |
Xia, X.-F.; Yu, J.; Wang, D. Adv. Synth. Catal. 2018, 360, 562.
doi: 10.1002/adsc.v360.3 |
| [12] |
Liu, Y.; Chen, X.-L.; Sun, K.; Li, X.-Y.; Zeng, F.-L.; Liu, X.-C.; Qu, L.-B.; Zhao, Y.-F.; Yu, B. Org. Lett. 2019, 21, 4019.
doi: 10.1021/acs.orglett.9b01175 |
| [13] |
Wang, S.-W.; Yu, J.; Zhou, Q.-Y.; Chen, S.-Y.; Xu, Z.-H.; Tang, S. ACS Sustainable Chem. Eng. 2019, 7, 10154.
doi: 10.1021/acssuschemeng.9b02178 |
| [14] |
Zhang, H.; Mou, X.; Chen, G.; He, G. Acta Chim. Sinica 2019, 77, 884. (in Chinese).
doi: 10.6023/A19060220 |
|
(张衡, 牟学清, 陈弓, 何刚, 化学学报, 2019, 77, 884.)
doi: 10.6023/A19060220 |
|
| [15] |
Li, D.; Wang, Y.; Jia, Z.; Ou, Z.; Dong, Y.; Lv, C.; Fu, G.; Liang, D. Eur. J. Org. Chem. 2019,4797.
|
| [16] |
Xiong, H.; Ramkumar, N.; Chiou, M.-F.; Jian, W.; Li, Y.; Su, J.-H.; Zhang, X.; Bao, H. Nat. Commun. 2019, 10, 122.
doi: 10.1038/s41467-018-07985-2 |
| [17] |
Wang, R.; Guan, W.; Han, Z.-B.; Liang, F.; Suga, T.; Bi, X.; Nishide, H. Org. Lett. 2017, 19, 2358.
doi: 10.1021/acs.orglett.7b00894 |
| [18] |
Fu, Q.; Wang, R.; Liang, F.; Guan, W. Org. Biomol. Chem. 2018, 16, 8950.
doi: 10.1039/C8OB02749H |
| [19] |
Chu, X.-Q.; Cheng, B.-Q.; Zhang, Y.-W.; Ge, D.; Shen, Z.-L.; Loh, T.-P. Chem. Commun. 2018, 54, 2615.
doi: 10.1039/C7CC09571F |
| [20] |
Chu, X.-Q.; Xie, T.; Li, L.; Ge, D.; Shen, Z.-L.; Loh, T.-P. Org. Lett. 2018, 20, 2749.
doi: 10.1021/acs.orglett.8b00963 |
| [21] |
Chu, X.-Q. Ge, D.; Wang, M.-L.; Rao, W.; Loh, T.-P.; Shen, Z.-L. Adv. Synth. Catal. 2019, 361, 4082.
doi: 10.1002/adsc.v361.17 |
| [22] |
Wang, R.; Wang, L.; Xu, Q.; Ren, B.-Y.; Liang, F. Org. Lett. 2019, 21, 3072.
doi: 10.1021/acs.orglett.9b00655 |
|
Chen, Y.; Li, L.; He, X.; Li, Z. ACS Catal. 2019, 9, 9098.
doi: 10.1021/acscatal.9b03189 |
| [1] | 张素珍, 张文文, 杨慧, 顾庆, 游书力. 铑催化2-烯基苯酚与炔烃的对映体选择性螺环化反应[J]. 有机化学, 2023, 43(8): 2926-2933. |
| [2] | 陈玉琢, 孙红梅, 王亮, 胡方芝, 李帅帅. 基于α-氢迁移策略构建杂环骨架的研究进展[J]. 有机化学, 2023, 43(7): 2323-2337. |
| [3] | 黄芬, 罗维纬, 周俊. 基于C—H键断裂的多氯烷基化反应研究进展[J]. 有机化学, 2023, 43(7): 2368-2390. |
| [4] | 田钰, 张娟, 高文超, 常宏宏. 二甲亚砜作为甲基化试剂在有机合成中的应用[J]. 有机化学, 2023, 43(7): 2391-2406. |
| [5] | 孙李星, 孙婷婷, 王海清, 吴淑芳, 王小烨, 刘天雅, 张宇辰. Lewis酸催化下3-烷基-2-吲哚烯与α,β-不饱和N-磺酰基亚胺的[2+4]环化反应[J]. 有机化学, 2023, 43(6): 2178-2188. |
| [6] | 任志军, 罗维纬, 周俊. 银介导的N-芳基丙烯酰胺串联环化反应研究进展[J]. 有机化学, 2023, 43(6): 2026-2039. |
| [7] | 李靖鹏, 黄顺桃, 杨棋, 李伟强, 刘腾, 黄超. 利用连续流动技术合成(Z)-N-乙烯基取代N,O-缩醛[J]. 有机化学, 2023, 43(4): 1550-1558. |
| [8] | 南江, 黄冠杰, 胡岩, 王波. 钌催化喹唑啉酮与碳酸亚乙烯酯的C—H [4+2]环化反应[J]. 有机化学, 2023, 43(4): 1537-1549. |
| [9] | 王海清, 杨爽, 张宇辰, 石枫. 邻羟基苄醇参与的催化不对称反应研究进展[J]. 有机化学, 2023, 43(3): 974-999. |
| [10] | 赵金晓, 魏彤辉, 柯森, 李毅. 可见光催化合成二氟烷基取代的多环吲哚化合物[J]. 有机化学, 2023, 43(3): 1102-1114. |
| [11] | 南宁, 吴双, 秦景灏, 李金恒. 基于硅烷化启动的环化反应研究进展[J]. 有机化学, 2023, 43(10): 3414-3453. |
| [12] | 桑田, 贾帆, 何静, 李春天, 刘岩, 刘平. I2催化β-酮腈与1H-吡唑-5-胺的环化反应[J]. 有机化学, 2023, 43(1): 195-201. |
| [13] | 刘东汉, 鲁席杭, 柴张梦洁, 杨浩琦, 孙瑜琳, 余富朝. 构建2H-吡咯-2-酮骨架的研究进展[J]. 有机化学, 2023, 43(1): 57-73. |
| [14] | 王川川, 马志伟, 侯学会, 杨龙华, 陈亚静. N-Ts氰胺在有机合成中的研究与应用[J]. 有机化学, 2023, 43(1): 74-93. |
| [15] | 覃小婷, 邹宁, 农彩梅, 莫冬亮. 九元氮杂环化合物合成最新研究进展[J]. 有机化学, 2023, 43(1): 130-155. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||