有机化学 ›› 2021, Vol. 41 ›› Issue (9): 3379-3389.DOI: 10.6023/cjoc202105036 上一篇 下一篇
综述与进展
收稿日期:2021-05-21
修回日期:2021-06-09
发布日期:2021-06-22
通讯作者:
王鹏
基金资助:
Peng Wanga( ), Da Yangb, Huan Liub
), Da Yangb, Huan Liub
Received:2021-05-21
Revised:2021-06-09
Published:2021-06-22
Contact:
Peng Wang
Supported by:文章分享

1,3-丁二烯是石脑油裂解过程C4馏分中最主要的组分, 通过羰基化反应合成的下游产品己二醛、己二酸、己二酸二甲酯在尼龙、增塑剂、医药中间体等的生产上有重要应用. 但是如何高效高化学/区域选择性合成这类高附加值产品一直是有机化学研究以及工业化生产的难题. 综述了近年来1,3-二烯类化合物(特别是1,3-丁二烯)发生羰基化反应(氢甲酰化反应、氢酯化反应、氢羧基化反应)构建高附加值化学品的研究进展, 并对该方法存在的难点以及未来发展方向进行了阐述和展望.
王鹏, 杨妲, 刘欢. 1,3-二烯烃的羰基化反应研究进展[J]. 有机化学, 2021, 41(9): 3379-3389.
Peng Wang, Da Yang, Huan Liu. Recent Advances on Carbonylation of 1,3-Dienes[J]. Chinese Journal of Organic Chemistry, 2021, 41(9): 3379-3389.

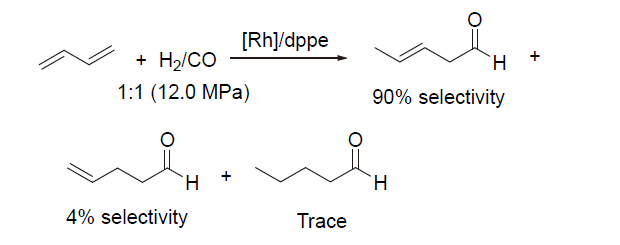


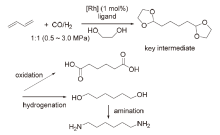





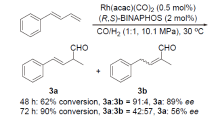
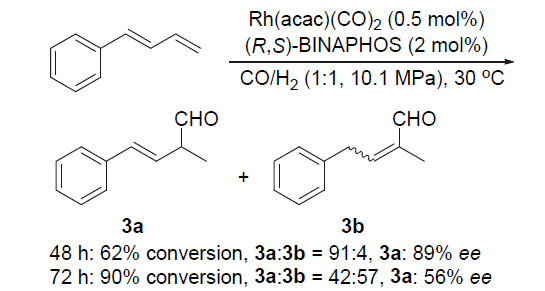
| [1] |
Nienburg, H. J.; Kummer, R.DE 2317625, 1974.
|
| [2] |
Mirbach, M. Transition Met. Chem. 1984, 9, 465.
doi: 10.1007/BF00620679 |
| [3] |
Herrmann, N.; Vogelsang, D.; Behr, A.; Seidensticker, T. ChemCatChem 2018, 10, 5342.
doi: 10.1002/cctc.v10.23 |
| [4] |
Börner, A.; Franke, R. Hydroformylation: Fundamentals, Processes, and Applications in Organic Synthesis, Wiley-VCH, Weinheim, 2016.
|
| [5] |
Klosin, J.; Landin, C. R. Acc. Chem. Res. 2007, 40, 1251.
doi: 10.1021/ar7001039 |
| [6] |
Fell, B.; Hermanns, P.US 5434312, 1995.
|
| [7] |
Briggs, J.; Packett, D.; Bryant, D.; Phillips, A.; Schreck, D.; Olson, K.; Tjaden, E.; Guram, A.; Eisenschmid, T.; Bragham, E.WO 9740001, 1997.
|
| [8] |
Packett, D. L. (to Union Carbide Coatings Service Technology Corp.) US 5312996, 1994.
|
| [9] |
Fell, B.; Rupilius, W. Tetrahedron Lett. 1969, 10, 2721.
doi: 10.1016/S0040-4039(01)88252-6 |
| [10] |
van Leeuwen, P.; Roobeek, C. F. J. Mol. Catal. 1985, 31, 345.
doi: 10.1016/0304-5102(85)85117-8 |
| [11] |
Fell, B.; Hermanns, P.; Bahrmann, H. J. Prakt. Chem. 1998, 340, 459.
|
| [12] |
Packett, D. L.; Briggs, J. R.; Bryant, D. R.; Phillips, A. G. WO 1997040003, 1997.
|
| [13] |
Fell, B.; Bahrmann, H. J. Mol. Catal. 1980, 8, 329.
doi: 10.1016/0304-5102(80)80074-5 |
| [14] |
Fell, B.; Rupilius, W. Tetrahedron Lett. 1969, 32, 2721.
|
| [15] |
van Leeuwen, P. W. N. M.; Roobeek, C. F. J. Mol. Catal. 1985, 31, 345.
doi: 10.1016/0304-5102(85)85117-8 |
| [16] |
Fyhr, C.; Garland, M. Organometallics 1993, 12, 1753.
doi: 10.1021/om00029a036 |
| [17] |
Liu, G.; Garland, M. J. Organomet. Chem. 2000, 608, 76.
|
| [18] |
Kummer, R.; Schneider, W.; Weiss, F. J. (to BASF AG).DE 2741511, 1979.
|
| [19] |
Kummer, R.(to BASF AG) DE 2414253, 1976.
|
| [20] |
Roobeek, C. F. (to Shell Internationale Research Maatschappij B. V.) EP 0033554, 1981.
|
| [21] |
van Leeuwen, P. W. N. M.; Roobeek, C. F. J. Mol. Catal. 1985, 31, 345.
doi: 10.1016/0304-5102(85)85117-8 |
| [22] |
Packett, D. L. (to Union Carbide Coatings Service Technology Corp. ) EP 577042, 1993.
|
| [23] |
Kummer, R.; Weiss, F. J. Proc.-Symp. Rhodium Homogeneous Catal. 1978, 87.
|
| [24] |
Kummer, R.(to BASF AG) DE 2414253, 1975.
|
| [25] |
Packett, D. L. (to Union Carbide Chemicals & Plastics)US 5312966, 1994.
|
| [26] |
Smits, H. A.; Spronken, J. M. H.; Wolters, H. F. W.; Boogers, J. A. F. (to DSMN. V.) EP 1223155, 2001.
|
| [27] |
Bertozzi, S.; Campigli, N.; Vitulli, G.; Lazzaroni, R.; Salvadori, P. J. Organomet. Chem. 1995, 487, 41.
|
| [28] |
Ohgomori, Y.; Suzuki, N.; Sumitani, N. J. Mol. Catal. A: Chem. 1998, 133, 289.
doi: 10.1016/S1381-1169(98)00129-0 |
| [29] |
Briggs, J. R.; Packett, D. L.; Bryant, D. R.; Phillips, A. G.; Schreck, D. J.; Guram, A. S.; Olson, K. D.; Eisenschmid, T. C.; Tjaden, E. B. (to Union Carbide Chemicals & Plastics Technology Corporation) US 6187970, 2001.
|
| [30] |
Huo, C. F.; Li, Y. W.; Beller, M.; Jiao, H. J. Organometallics 2005, 24, 3634.
doi: 10.1021/om0500422 |
| [31] |
Smith, S. E.; Rosendahl, T.; Hofmann, P. Organometallics 2011, 30, 3643.
doi: 10.1021/om200334g |
| [32] |
Schmidt, S.; Barath, E.; Prommnitz, T.; Rosendahl, T.; Rominger, F.; Hofmann, P. Organometallics 2014, 33, 6018.
doi: 10.1021/om500643t |
| [33] |
Schmidt, S.; Deglmann, P.; Hofmann, P. ACS Catal. 2014, 4, 3593.
doi: 10.1021/cs500718v |
| [34] |
Schmidt, S.; Barath, E.; Larcher, C.; Rosendahl, T.; Hofmann, P. Organometallics 2015, 34, 841.
doi: 10.1021/om501015z |
| [35] |
Maji, T.; Mendis, C. H.; Thompson, W. H.; Tunge, J. A. J. Mol. Catal. A: Chem. 2016, 424, 145.
doi: 10.1016/j.molcata.2016.08.021 |
| [36] |
Mormul, J.; Breitenfeld, J.; Trapp, O.; Paciello, R.; Schaub, T.; Hofmann, P. ACS Catal. 2016, 6, 2802.
doi: 10.1021/acscatal.6b00189 |
| [37] |
Yua, S. M.; Snavelya, W. K.; Chaudharia, R. V.; Subramaniam, B. Mol. Catal. 2020, 484, 110721.
|
| [38] |
Tenorio, M. J.; Chaudhari, R. V.; Subramaniam, B. Ind. Eng. Chem. Res. 2019, 58, 22526.
doi: 10.1021/acs.iecr.9b05184 |
| [39] |
Musser, M. T. Ullmanns Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2000.
|
| [40] |
Alini, S.;Babini P. Handbook of Advanced Methods and Processes in Oxidation Catalysis, Imperial College Press, London, 2014.
|
| [41] |
Zachry, J. B.; Aldridge, C. L. US 3161672, 1964.
|
| [42] |
Von, K. N. US 3876695, 1975.
|
| [43] |
Isogai, Y.; Hosokawa, M.; Ookawa, T.; Wakui, N.; Watanabe, T.JP 5788147, 1982.
|
| [44] |
Hsu, C. K.; Dobinson, F.US 4575562, 1986.
|
| [45] |
Maerkl, R.US 4777284, 1988.
|
| [46] |
Drent, E.; Van, G. J. US 4861912, 1989.
|
| [47] |
Omatsu, T.; Tokito, Y.JP 04208247, 1992.
|
| [48] |
Atadan, E. M.; Bruner, H. S. US 5292944, 1994.
|
| [49] |
Matsuda, A.; Fujii, T.JP 06192175, 1994.
|
| [50] |
Denis, P.; Patois, C.; Perron, R.EP 0648731, 1995.
|
| [51] |
Sielcken, O. E.; Hovenkamp, H.WO 9506027, 1995.
|
| [52] |
Poole, A. D.; Sunley, J. G. UK 2327420, 1999.
|
| [53] |
Drent, E.; Jager, W. W. WO 00056695, 2000.
|
| [54] |
Sielcken, O. E. WO 0121569, 2001.
|
| [55] |
Yang, J.; Liu, J. W.; Neumann, H.; Franke, R.; Jackstell, R.; Beller, M. Science 2019, 366, 1514.
doi: 10.1126/science.aaz1293 pmid: 31857484 |
| [56] |
Yang, J.; Liu, J. W.; Ge, Y.; Huang, W. H.; Ferretti, F.; Neumann, H.; Jiao, H. J.; Franke, R.; Jackstell, R.; Beller, M. Angew. Chem. Int. Ed. 2021, 60, 2.
doi: 10.1002/anie.v60.1 |
| [57] |
Skoog, E.; Shin, J. H.; Saez-Jimenez, V.; Mapelli, V.; Olsson, L. Biotechnol. Adv. 2018, 36, 2248.
doi: S0734-9750(18)30175-7 pmid: 30389426 |
| [58] |
Gunukula, S.; Anex, R. P. Biofuels, Bioprod. Biorefin. 2017, 11, 897.
|
| [59] |
Bart, J. C. J.; Cavallaro, S. Ind. Eng. Chem. Res. 2015, 54, 1.
doi: 10.1021/ie5020734 |
| [60] |
Eia, US.Annual Energy Outlook 2015: with Projections to 2040 2017.
|
| [61] |
Eggleston, S.; Buendia, L.; Miwa, K.; Ngara, T.; Tanabe, K. Institute for Global Environmental Strategies (Japó). 2006 IPCC Guidelines for National Greenhouse Gas Inventories, IGES, Japan, 2006.
|
| [62] |
Agency USEP Inventory of US Greenhouse Gas Emissions and Sinks: 1990-2015, US Environmental Protection Agency, Washington, DC, 2017.
|
| [63] |
Lewis, Sr. R. L. Carcinogenically Active Chemicals, Van Nostrand Reinhold Company, New York, 1990.
|
| [64] |
Smith, M. T.; Jones, R. M.; Smith, A. H. Cancer Epidemiol. Biomarkers Prev. 2007, 16. 385.
doi: 10.1158/1055-9965.EPI-06-1057 |
| [65] |
Rios, J.; Lebeau, J.; Yang, T.; Li, S.; Lynch, M. D. Green Chem. 2021, 23, 3172.
doi: 10.1039/D1GC00638J |
| [66] |
Tortajada, A.; Ninokata, R.; Martin, R. J. Am. Chem. Soc. 2018, 140, 2050.
doi: 10.1021/jacs.7b13220 pmid: 29376353 |
| [67] |
Trzeciak, A. M.; Ziółkowski, J. J. J. Organomet. Chem. 1994, 479, 213.
|
| [68] |
Adkins, H.; Williams, J. L. R.; J. Org. Chem. 1952, 17, 980.
doi: 10.1021/jo50007a012 |
| [69] |
Barros, H. J. V.; Guimãraes, C. C.; dos Santos, E. N.; Gusevskaya, E. V. Catal. Commun. 2007, 8, 747.
doi: 10.1016/j.catcom.2006.09.015 |
| [70] |
Barros, H. J. V.; Guimãraes, C. C.; dos Santos, E. N.; Gusevskaya, E. V. Organometallics 2007, 26, 2211.
doi: 10.1021/om060994n |
| [71] |
Behr, A.; Reyer, S.; Tenhumberg, N. Dalton Trans. 2011, 40, 11742.
doi: 10.1039/c1dt11292a |
| [72] |
Yu, S.; Chie, Y. M.; Zhang, X.; Dai, L.; Zhang, X. Tetrahedron Lett. 2009, 50, 5575.
doi: 10.1016/j.tetlet.2009.07.066 |
| [73] |
Morikawa, M. Bull. Chem. Soc. Jpn. 1964, 37, 379.
doi: 10.1246/bcsj.37.379 |
| [74] |
Watkins, A. L.; Landis, C. R. Org. Lett. 2011, 13, 164.
doi: 10.1021/ol102797t pmid: 21133397 |
| [75] |
Horiuchi, T.; Ohta, T.; Shirakawa, E.; Nozaki, K.; Takaya, H. Tetrahedron 1997, 53, 7795.
doi: 10.1016/S0040-4020(97)00471-7 |
| [76] |
Horiuchi, T.; Ohta, T.; Nozaki, K.; Takaya, H. Chem. Commun. 1996, 2, 155.
|
| [77] |
Adint, T. T.; Wong, G. W.; Landis, C. R. J. Org. Chem. 2013, 78, 4231.
doi: 10.1021/jo400525w |
| [78] |
Adkins, H.; Williams, J. L. R. J. Org. Chem. 1952, 17, 980.
doi: 10.1021/jo50007a012 |
| [79] |
Meyer, W. Hydrocarbon Process., nt. Ed. 1976, 194, 235.
|
| [80] |
Morgan, M. Chem. Ind. (London) 1999, 645.
|
| [81] |
Cheung, T. T. P. Cyclopentadiene and Dicyclopentadiene, in Kirk-Othmer Encyclopedia of Chemical Technology, Wiley-VCH Verlag GmbH, Weinheim, 2004.
|
| [82] |
Hönicke, D.; Födisch, R.; Claus, P.; Olson, M. Ullmann's Encyclopedia of Industrial Chemistry, 7th ed., Wiley-VCH, Weinheim, 2005, p. 1-14.
|
| [83] |
Behr, A.; Levikov, D.; Vogelsang, D. J. Mol. Catal. A: Chem. 2015, 406, 114.
doi: 10.1016/j.molcata.2015.06.003 |
| [84] |
Neubert, P.; Fuchs, S.; Behr, A. Green Chem. 2015, 17, 4045.
doi: 10.1039/C5GC00020C |
| [1] | 廖旭, 王泽宇, 唐武飞, 林金清. 多孔有机聚合物用于化学固定二氧化碳的研究进展[J]. 有机化学, 2023, 43(8): 2699-2710. |
| [2] | 刘双, 邹亮华, 王晓明. 均相钴催化氨硼烷的脱氢及转移氢化反应的研究进展[J]. 有机化学, 2023, 43(5): 1713-1725. |
| [3] | 莫百川, 陈春霞, 彭进松. 木质素及其衍生物负载金属催化剂在有机合成中的应用研究进展[J]. 有机化学, 2023, 43(4): 1215-1240. |
| [4] | 苏沛锋, 倪金煜, 柯卓锋. 二氧化碳硅氢化及相关转化的均相催化体系研究进展[J]. 有机化学, 2023, 43(10): 3526-3543. |
| [5] | 刘欢, 林旭锋, 杨妲. 新型铱配合物催化水作氢源的氢甲酰化反应[J]. 有机化学, 2021, 41(9): 3571-3577. |
| [6] | 陈鑫, 陈春霞, 彭进松. 纤维素及其衍生物负载铜催化有机反应的研究进展[J]. 有机化学, 2021, 41(4): 1319-1336. |
| [7] | 黄文斌, 邱丽琪, 任方煜, 何良年. 过渡金属催化CO2氢化反应研究进展[J]. 有机化学, 2021, 41(10): 3914-3934. |
| [8] | 王飞雨, 张志朋, 黄菲. 基于重氮酯的O—H插入反应研究进展[J]. 有机化学, 2021, 41(1): 144-157. |
| [9] | 刘嘉豪, 韩静杰, 易小艺, 刘超, 何飘. 甲酸分解制氢均相催化剂的研究进展[J]. 有机化学, 2020, 40(9): 2658-2668. |
| [10] | 宗玲博, 陈建宾, 任新意, 张国营, 贾肖飞. 有机聚合物负载铑催化剂在氢甲酰化反应中的应用研究进展[J]. 有机化学, 2020, 40(8): 2308-2321. |
| [11] | 徐子悦, 罗驿, 王辉, 张丹维, 黎占亭. 有机多孔聚合物非均相催化可见光诱导有机转化[J]. 有机化学, 2020, 40(11): 3777-3793. |
| [12] | 张翔, 郭彩红, 武海顺. 甲醇低温脱氢均相过渡金属催化剂研究进展[J]. 有机化学, 2019, 39(9): 2458-2466. |
| [13] | 吕小妹, 阮建成, 陈新志, 钱超. 交联壳聚糖微球负载铜水相催化Ullmann反应[J]. 有机化学, 2019, 39(6): 1720-1726. |
| [14] | 徐鹏, 段新红. 以水为溶剂的铃木-宫浦偶联反应最新研究进展[J]. 有机化学, 2019, 39(12): 3315-3327. |
| [15] | 侯亚东, 庞海霞, 杨超, 惠永海. 介孔分子筛MCM-41固载席夫碱与Cu(ClO4)2·6H2O催化合成螺[吲哚-噻唑啉酮]衍生物[J]. 有机化学, 2018, 38(8): 2036-2044. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||