有机化学 ›› 2021, Vol. 41 ›› Issue (10): 4021-4027.DOI: 10.6023/cjoc202105059 上一篇 下一篇
所属专题: 南开大学化学学科创立100周年; 热点论文虚拟合集
研究论文
收稿日期:2021-05-31
修回日期:2021-07-07
发布日期:2021-07-19
通讯作者:
谢建华
基金资助:
Huaiyu Bin, Li Cheng, Xiaohui Yang, Jianhua Xie( ), Qilin Zhou
), Qilin Zhou
Received:2021-05-31
Revised:2021-07-07
Published:2021-07-19
Contact:
Jianhua Xie
Supported by:文章分享

报道了一种不对称构建石杉碱甲及其类似物吡啶稠合手性双环[3.3.1]壬烷骨架的策略. 该策略主要通过具有环外双键的γ,δ-不饱和β-酮酸酯的动态动力学拆分不对称催化氢化, 并结合分子内芳基化反应为关键步骤来不对称构建吡啶稠合手性双环[3.3.1]壬烷骨架. 依据此策略, 从已知的原料出发, 经5步反应以17.4%的总收率完成了石杉碱甲及其类似物核心手性骨架结构的简洁、快速不对称构建.
宾怀玉, 程立, 杨小会, 谢建华, 周其林. 石杉碱甲及其类似物吡啶稠合手性双环[3.3.1]壬烷骨架的不对称构建[J]. 有机化学, 2021, 41(10): 4021-4027.
Huaiyu Bin, Li Cheng, Xiaohui Yang, Jianhua Xie, Qilin Zhou. Enantioselective Construction of the Pyridine-Fused Chiral Bicyclo- [3.3.1]nonane Skeleton of Huperzine A and Its Analogues[J]. Chinese Journal of Organic Chemistry, 2021, 41(10): 4021-4027.
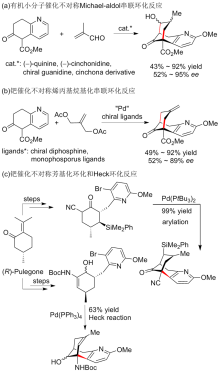

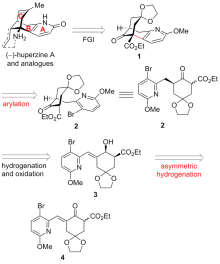

| [1] |
(a) Siengalewicz, P.; Mulzer, J.; Rinner, U. In The Alkaloids: Chemistry and Biology, Ed.: Knölker, H.-J., Academic Press, Cambridge, MA, 2013, Vol. 72, pp. 1-151.
|
|
(b) Ma, X. Q.; Gang, D. R. Nat. Prod. Rep. 2004, 21, 752.
doi: 10.1039/b409720n |
|
|
(c) Xiao, C.; Gao, L.; Wang, J.; Miao, Y.; Fan, H. Chin. J. Org. Chem. 2017, 37, 810. (in Chinese)
doi: 10.6023/cjoc201611032 |
|
|
(肖春霞, 曹林, 王佳, 苗银龙, 范华芳, 有机化学, 2017, 37, 810.)
doi: 10.6023/cjoc201611032 |
|
| [2] |
(a) Herzon, S. B.; Tun, M. K. M. J. Exp. Pharmacol. 2012, 4, 113.
|
|
(b) Qian, Z. M.; Ke, Y. Front. Aging Neurosci. 2014, 6, 216.
|
|
| [3] |
(a) Wang, L.-L.; Hao, L.-J.; Zhou, Z.-B.; Zhu, X.-L.; Shi, Z.-H.; Miyamoto, T.; Pan, K. Phytochemistry 2018, 154, 63.
doi: 10.1016/j.phytochem.2018.06.016 pmid: 23941108 |
|
(b) Zhang, D.-B.; Chen, J.-J.; Song, Q.-Y.; Zhang, L.; Gao, K. Molecules 2014, 19, 9999.
doi: 10.3390/molecules19079999 pmid: 23941108 |
|
|
(c) Tang, Y.; Fu, Y.; Xiong, J.; Li, M.; Ma, G.-L.; Yang, G.-X.; Wei, B.-G.; Zhao, Y.; Zhang, H.-Y.; Hu, J.-F. J. Nat. Prod. 2013, 76, 1475.
doi: 10.1021/np4003355 pmid: 23941108 |
|
|
(d) Yin, S.; Fan, C.-Q.; Wang, X.-N.; Yue, J.-M. Helv. Chem. Acta 2006, 89, 138.
doi: 10.1002/(ISSN)1522-2675 pmid: 23941108 |
|
| [4] |
(a) Bai, D.-L.; Tang, X.-C.; He, X.-C. Curr. Med. Chem. 2000, 7, 355.
pmid: 10637369 |
|
(b) Ma, X.; Tan, C.; Zhu, D.; Gang, D.-R. J. Ethnopharmacol. 2006, 104, 54.
doi: 10.1016/j.jep.2005.08.042 pmid: 10637369 |
|
| [5] |
Zheng, S.; Yu, C.; Shen, Z. Chin. J. Org. Chem. 2013, 33, 2261. (in Chinese)
doi: 10.6023/cjoc201306006 |
|
(郑书岩, 郁春辉, 沈征武, 有机化学, 2013, 33, 2261.)
doi: 10.6023/cjoc201306006 |
|
| [6] |
Qian, L.-G.; Ji, R.-Y. Tetrahedron Lett. 1989, 30, 2089.
doi: 10.1016/S0040-4039(01)93719-0 |
| [7] |
Xia, Y.; Kozikowshi, A. P. J. Am. Chem. Soc. 1989, 111, 4166.
doi: 10.1021/ja00194a004 |
| [8] |
(a) Chen, W.-P.; Yang, F.-Q. Chin. J. Med. Chem. 1995, 15, 10. (in Chinese)
|
|
(陈卫平, 杨福秋, 中国药物化学杂志, 1995, 15, 10.)
|
|
|
(b) Kaneko, S.; Yoshino, T.; Katoh, T.; Terashima, S. Heterocycles 1997, 46, 27.
doi: 10.3987/COM-96-S4 |
|
|
(c) Kaneko, S.; Yoshino, T.; Katoh, T.; Terashima, S. Tetrahedron 1998, 54, 5471.
doi: 10.1016/S0040-4020(98)00227-0 |
|
|
(d) Pan, Q.-B.; Ma, D.-W. Chin. J. Chem. 2003, 21, 793.
doi: 10.1002/cjoc.20030210716 |
|
|
(e) Ding, X.-H.; Li, X.; Liu, D.; Cui, W.-C.; Ju, X.; Wang, S.-Z.; Yao, Z.-J. Tetrahedron 2012, 68, 6240.
doi: 10.1016/j.tet.2012.05.061 |
|
| [9] |
(a) Kozikowski, A.P.; Campiani, G.; Aagaard, P.; Mckinney, M. J. Chem. Soc., hem. Commun. 1993, 10, 860.
|
|
(b) Campiano, G.; Sun, L. Q.; Kozikowski, A. P. J. Org. Chem. 1993, 58, 7660.
doi: 10.1021/jo00079a008 |
|
| [10] |
(a) Kaneko, S.; Yoshino, T.; Katoh, T.; Terashima, S. Tetrahedron: Asymmetry 1997, 8, 829.
doi: 10.1016/S0957-4166(97)00048-7 |
|
(b) He, X.-C.; Wang, B.; Bai, D.-L. Tetrahedron Lett. 1998, 39, 411.
doi: 10.1016/S0040-4039(97)10535-4 |
|
|
(c) Chassaing, C.; Hanudrechy, A.; Langlois, Y. Tetrahedron Lett. 1999, 40, 8805.
doi: 10.1016/S0040-4039(99)01874-2 |
|
|
(d) He, X.-C.; Wang, B.; Yu, G, Bai, D. Tetrahedron: Asymmetry 2001, 12, 3213.
doi: 10.1016/S0957-4166(02)00006-X |
|
|
(e) Tudhope, S. R.; Bellamy, J. A.; Ball, A.; Rajasekar, D.; Azadi-Ardakani, M.; Meera, H. S.; Gnanadeepam, J. M.; Saiganesh, R.; Gibson, F.; He, L.; Behrens, C. H.; Underiner, G.; Marfurt, J.; Favre, N. Org. Process Res. Dev. 2012, 16, 635.
doi: 10.1021/op200360b |
|
|
(f) Lin, C.-F.; Chien, C.-W.; Ojima, I. Org. Chem. Front. 2014, 1, 1062.
doi: 10.1039/C4QO00180J |
|
| [11] |
Tun, M. K. M.; Wüstmann, D.; Herzon, S. B. Chem. Sci. 2011, 2, 2251.
doi: 10.1039/c1sc00455g |
| [12] |
(a) Ding, R.; Sun, B.-F.; Lin, G.-Q. Org. Lett. 2012, 14, 4446.
doi: 10.1021/ol301951r pmid: 24299147 |
|
(b) Ding, R.; Fu, J.-G.; Xu, G.-Q.; Sun, B.-F.; Lin, G.-Q. J. Org. Chem. 2014, 79, 240.
doi: 10.1021/jo402419h pmid: 24299147 |
|
|
(c) Fu, J.-G.; Xu, G.-Q.; Ding, R.; Lin, G.-Q.; Sun, B.-F. Org. Chem. Front. 2016, 3, 62.
doi: 10.1039/C5QO00355E pmid: 24299147 |
|
| [13] |
Haley, H. M. S.; Payer, S. E.; Papiddocha, S. M.; Clemens, S.; Nyenhuis, J.; Sarpong, R. J. Am. Chem. Soc. 2021, 143, 4732.
doi: 10.1021/jacs.1c00457 |
| [14] |
(a) Xie, J.-H.; Zhou, Q.-L. Aldrichim. Acta 2015, 48, 33.
|
|
(b) Lin, H.; Xiao, L.-J.; Zhou, M.-J.; Yu, H.-M.; Xie, J.-H.; Zhou, Q.-L. Org. Lett. 2016, 18, 1434.
doi: 10.1021/acs.orglett.6b00369 |
|
|
(c) Liu, Y.; Cheng, L.-J.; Yue, H.-T.; Che, W.; Xie, J.-H.; Zhou, Q.-L. Chem. Sci. 2016, 6, 4725.
|
|
|
(d) Zou, X.-D.; Gou, S.-M.; Yang, R.; Xie, J.-H.; Zhou, Q.-L. Chem. Sci. 2017, 8, 6202.
doi: 10.1039/C7SC02112G |
|
|
(e) Liu, Y.-T.; Li, L.-P.; Xie, J.-H.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2017, 56, 12708.
doi: 10.1002/anie.v56.41 |
|
|
(f) Zou, X.-D.; Gou, S.-M.; Yang, R.; Xie, J.-H.; Zhou, Q.-L. Org. Lett. 2017, 19, 5240.
doi: 10.1021/acs.orglett.7b02517 |
|
|
(g) Yang, X.-H.; Gu, X.-S.; Bin, H.-Y; Xie, J.-H.; Zhou, Q.-L. Chin. J. Org. Chem. 2020, 40, 3963. (in Chinese)
doi: 10.6023/cjoc202007052 |
|
|
(杨小会, 顾雪松, 宾怀玉, 谢建华, 周其林, 有机化学, 2020, 40, 3963.)
doi: 10.6023/cjoc202007052 |
|
| [15] |
(a) Bin, H.-Y.; Wang, K.; Yang, D.; Yang, X.-H.; Xie, J.-H.; Zhou, Q.-L. Angew. Chem., Int. Ed. 2019, 58, 1174.
doi: 10.1002/anie.v58.4 |
|
(b) Bin, H.-Y.; Cheng, L.; Wu, X.; Zhu, C.-L.; Yang, X.-H.; Xie, J.-H.; Zhou, Q.-L. Chem. Sci. 2021, 12, 7793.
doi: 10.1039/D1SC02044G |
|
| [16] |
(a) Xie, J.-H.; Zhou, Q.-L. Acta Chim. Sinica 2014, 72, 778. (in Chinese)
doi: 10.6023/A14050364 |
|
(谢建华, 周其林, 化学学报, 2014, 72, 778.)
doi: 10.6023/A14050364 |
|
|
(b) Yang, X.-H.; Xie, J.-H.; Zhou, Q.-L. Org. Chem. Front. 2014, 1, 190.
doi: 10.1039/c3qo00056g |
|
|
(c) Xie, J.-H.; Liu, X.-Y.; Xie, J.-B.; Wang, L.-X.; Zhou, Q.-L. Angew. Chem., nt. Ed. 2011, 50, 7329.
|
|
| [17] |
(a) Lu, S.-M.; Bolm, C. Angew. Chem.,Int. Ed. 2008, 47, 8920.
doi: 10.1002/anie.v47:46 |
|
(b) Lu, W.-J.; Chen, Y.-W.; Hou, X.-L. Angew. Chem.,Int. Ed. 2008, 47, 10133.
doi: 10.1002/anie.v47:52 |
|
|
(c) Tian, F.; Yao, D.; Liu, Y.; Xie, F.; Zhang, W. Adv. Synth. Catal. 2010, 352, 1841.
doi: 10.1002/adsc.v352:11/12 |
|
|
(d) Liu, X.; Han, Z.; Wang, Z.; Ding, K. Angew. Chem., Int. Ed. 2014, 53, 1978.
|
| [1] | 陈东平, 杨春红, 李明, 赵国孝, 王文鹏, 王喜存, 权正军. 芳炔参与的三组分芳基化反应进展[J]. 有机化学, 2023, 43(2): 503-525. |
| [2] | 孟祥辉, 杨亮茹, 刘琪琳, 董振华, 袁金伟, 肖咏梅, 毛璞. 酰胺功能化吡啶/嘧啶螯合氮杂环卡宾钯化合物的合成、结构及其催化的咪唑C-5芳基化反应[J]. 有机化学, 2022, 42(11): 3747-3756. |
| [3] | 宋文越, 饶小峰, 卜庆青, 刘宁. 咔唑桥连NCN齿形钯配合物催化的唑类C-H键直接芳基化反应[J]. 有机化学, 2020, 40(2): 489-500. |
| [4] | 黄远婷, 陈迁. 芳炔参与的磷和硫芳基化反应研究进展[J]. 有机化学, 2020, 40(12): 4087-4100. |
| [5] | 王卫伟, 赵宇, 刘鑫磊, 耿瑞, 王明安. 通过(E)-7-甲基-2,6-辛二烯酸的立体选择性Mizoroki-Heck芳基化反应合成(E)-3-芳基-7-甲基-2,6-辛二烯酸[J]. 有机化学, 2019, 39(4): 1129-1135. |
| [6] | 肖祯, 乐强, 冉子垚, 张谦, 李栋. 铜促进的8-酰氨基喹啉类化合物N-芳基化反应[J]. 有机化学, 2018, 38(5): 1193-1198. |
| [7] | 邹浩, 王雪丁, 杨维清, 张园园, 陈华, 王玉良, 马梦林, 杜泉. 三聚氯氰与低沸点芳香环的Friedel-Crafts封管反应研究[J]. 有机化学, 2017, 37(10): 2697-2704. |
| [8] | 王沁婷, 赵帅, 金雷, 陈新. 相转移催化法和手性催化加氢法立体选择性地合成Fmoc保护的(S)-3,5-二溴苯丙氨酸[J]. 有机化学, 2016, 36(9): 2242-2246. |
| [9] | 袁乾家, 张万斌. 亚磷酰胺配体在铱催化不对称氢化反应中的应用[J]. 有机化学, 2016, 36(2): 274-282. |
| [10] | 余坤矫, 张振锋, 霍小红, 王兴广, 刘德龙, 张万斌. D-(R)-酪氨酸的不对称催化氢化合成及其在药物合成中的应用[J]. 有机化学, 2013, 33(9): 1932-1938. |
| [11] | 郑书岩, 郁春辉, 沈征武. 石杉碱甲的合成研究进展[J]. 有机化学, 2013, 33(11): 2261-2270. |
| [12] | 刘伟, 毕艳兰. 铜催化的芳环C—H 键直接芳基化反应研究进展[J]. 有机化学, 2012, 32(06): 1041-1050. |
| [13] | 马亚军, 严彪, 郭政科, 赵明涛, 韩海飞, 程芬芬, 葛靖国, 王金龙, 张岗虎. 吡啶氮桥联三唑卡宾反应性质及其Pd(II)-配合物催化杂芳环与芳基溴的α-烷基化反应研究[J]. 有机化学, 2011, 31(03): 368-373. |
| [14] | 马元辉,张勇健,张万斌. β-胺基丙烯酸酯衍生物的不对称催化氢化反应研究进展[J]. 有机化学, 2007, 27(03): 289-297. |
| [15] | 高宏飞,郭向前,杨刚,徐南平. 新型含氮三齿钯(II)配合物的合成及其催化性能研究[J]. 有机化学, 2007, 27(01): 134-137. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||